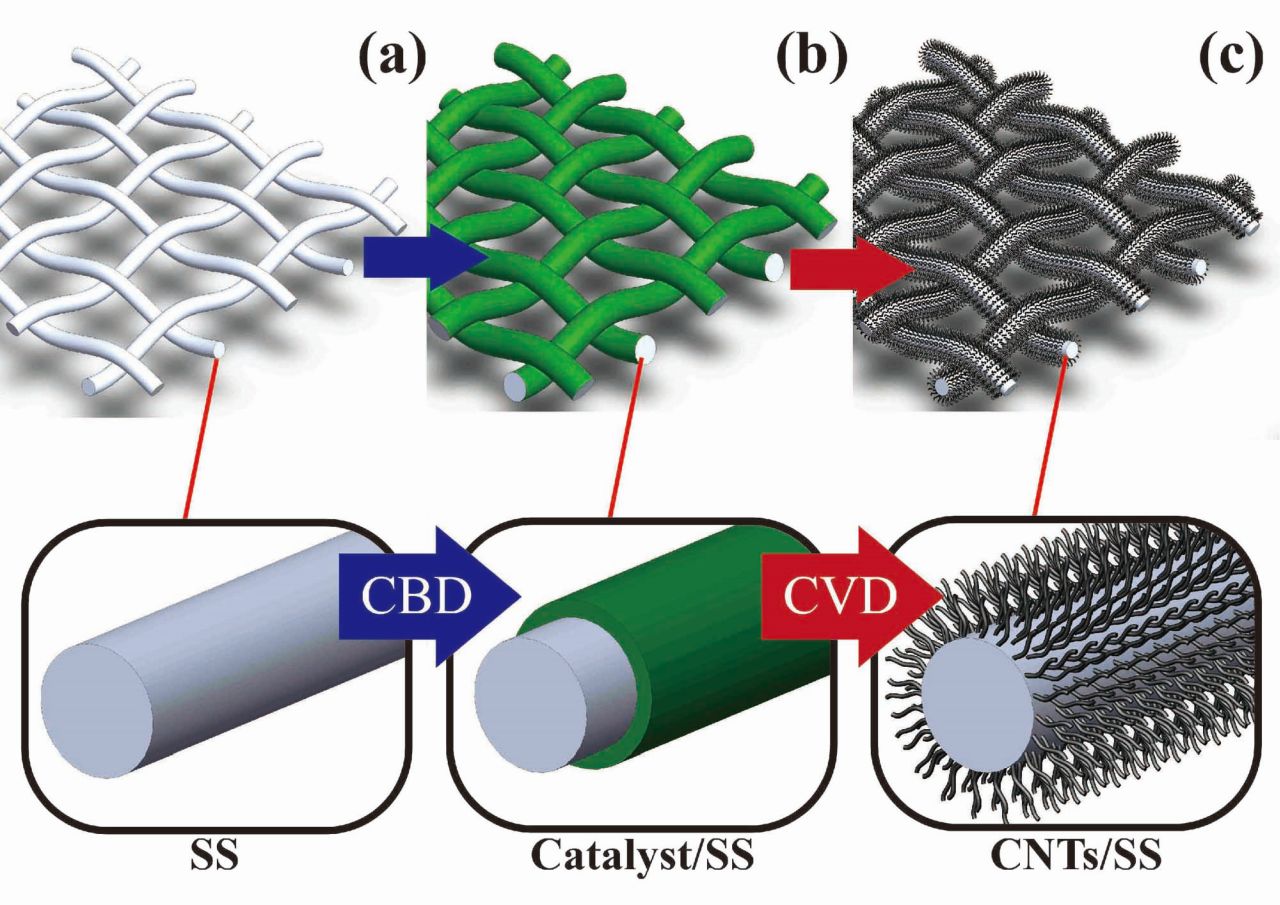
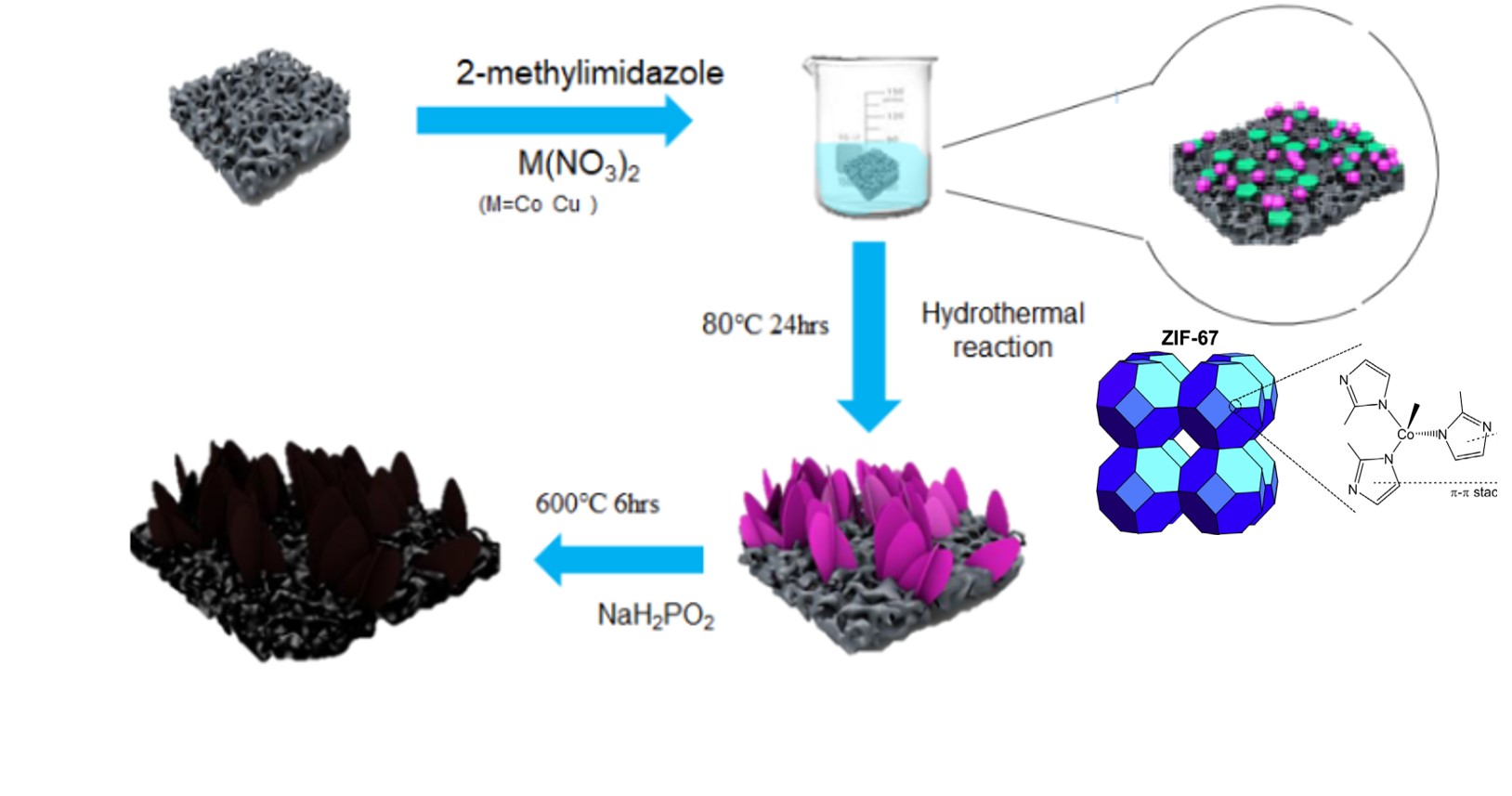
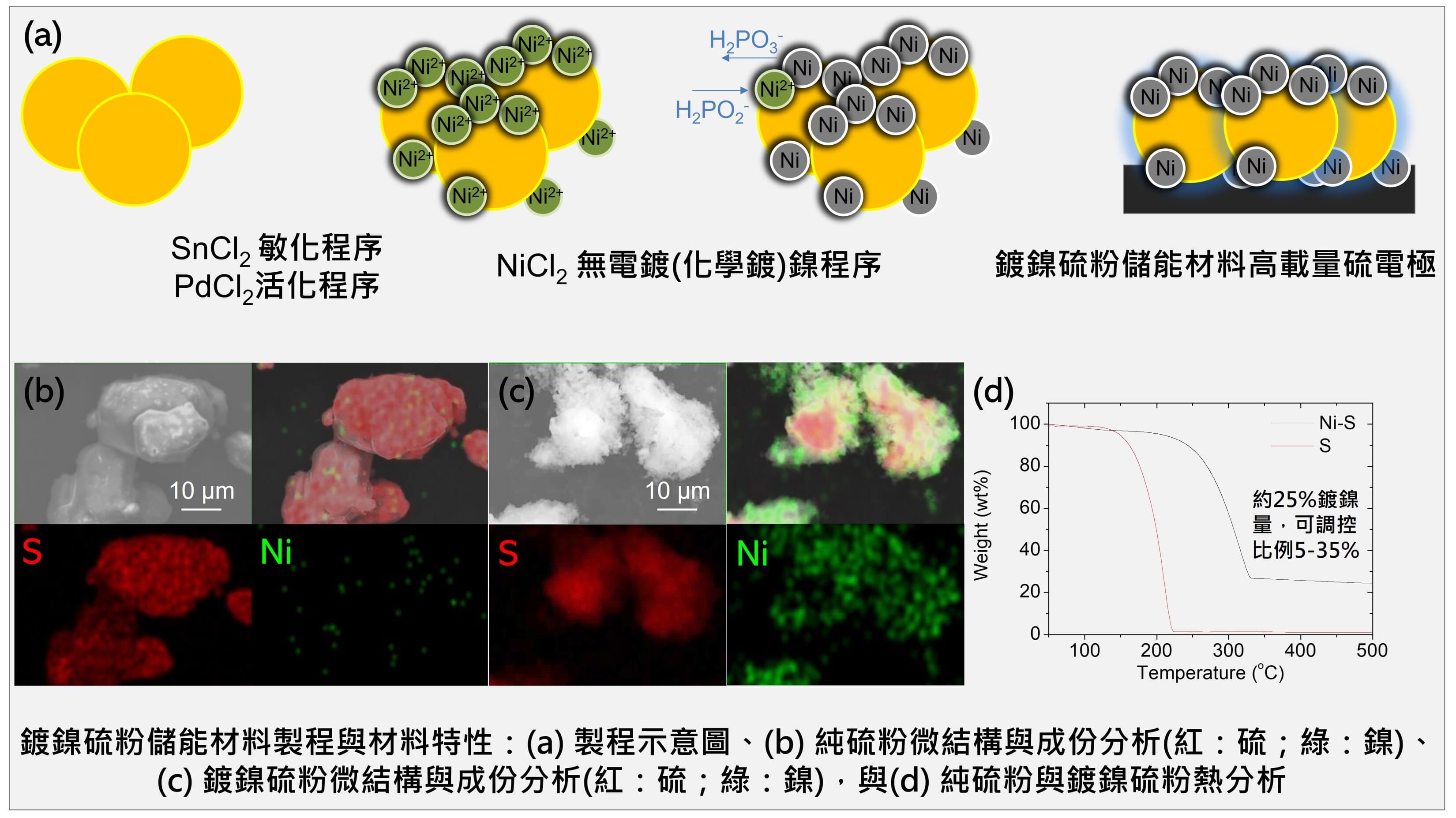
| Summary |
This technology utilizes the Pulsed Laser Irradiation Scanning on Mixed Salt Solutions method to develop a non-precious metal catalyst based on a Ni-Fe-Mn ternary alloy on metal porous materials, forming porous electrode structures. When applied to Anion Exchange Membrane Water Electrolyzers, it exhibits excellent performance. The technology is simple to operate, cost-effective, and fast to produce, enabling the rapid production of large-area, high-performance catalyst-coated porous electrodes. |
| Scientific Breakthrough |
Our technology deposits a NiFeMn ternary catalyst onto nickel foam. The overpotential is less than 150 mV at 10 mA/cm². In durability tests exceeding 1500 hours, the electrolysis efficiency degradation is less than 1%. The AEMWE achieves a high current density of 1800 mA/cm² at 2 V, with stable voltage during long-term operation. This performance meets the key performance indicators for advanced AEMWE as listed by the International Renewable Energy Agency (IRENA) in 2020. |
| Industrial Applicability |
Our team has developed an AEMWE technology that uses non-precious metal catalysts and anion exchange membranes. This approach significantly reduces the manufacturing costs of water electrolyzers and enhances environmental compatibility, making hydrogen production more affordable and sustainable. This is crucial for promoting the widespread adoption and commercialization of hydrogen technologies, facilitating the development and application of green energy. |