| Technical Name |
Application of a novel nucleic acid structure immune activation composition as adjuvant for nasal spray vaccines |
| Project Operator |
National Health Research Institutes |
| Project Host |
莊宗顯 |
| Summary |
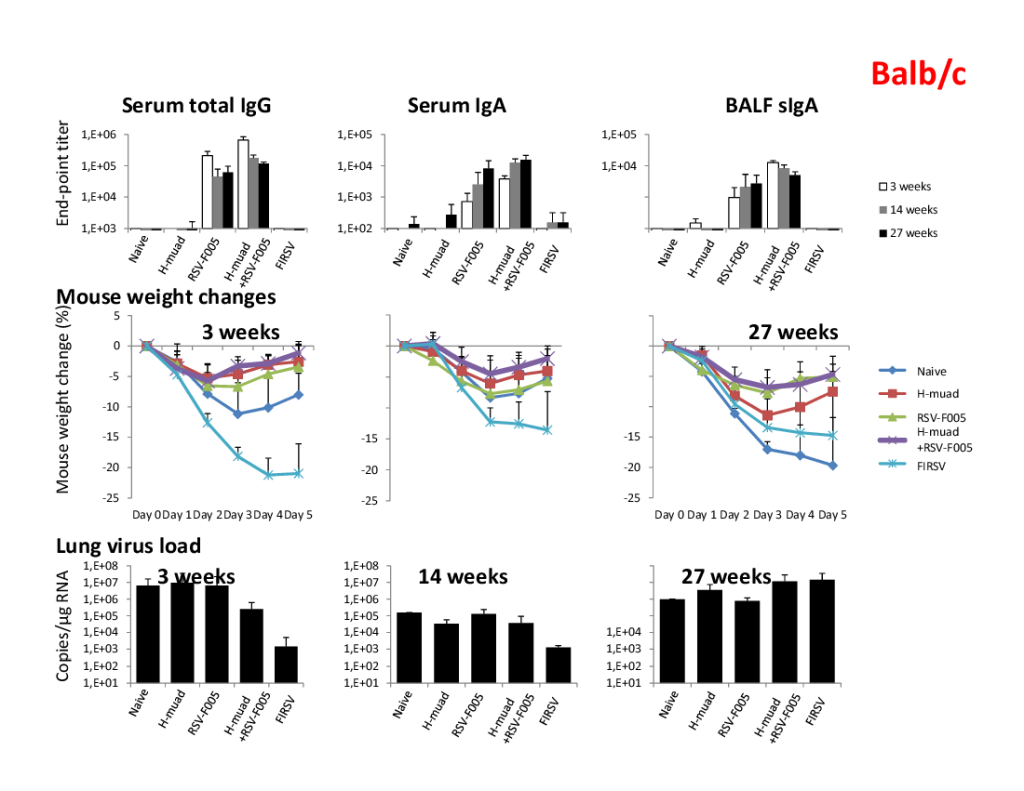
The combination of CpG-2722 and 2’3’-c-di-AM(PS)2 exhibited a cooperative adjuvant effect for SARS-CoV-2 RBD vaccines and a favorable safety profile in the nasal cavities of mice. CpG-2722/cyclic dinucleotide combinations enhanced the performance of nasal H7 influenza vaccines. Nasal administration of these combination-adjuvanted shingles vaccines elicited a superior vaccine response compared to intramuscularly injected Shingrix, indicating the superior adjuvant activity of these combinations. |
| Scientific Breakthrough |
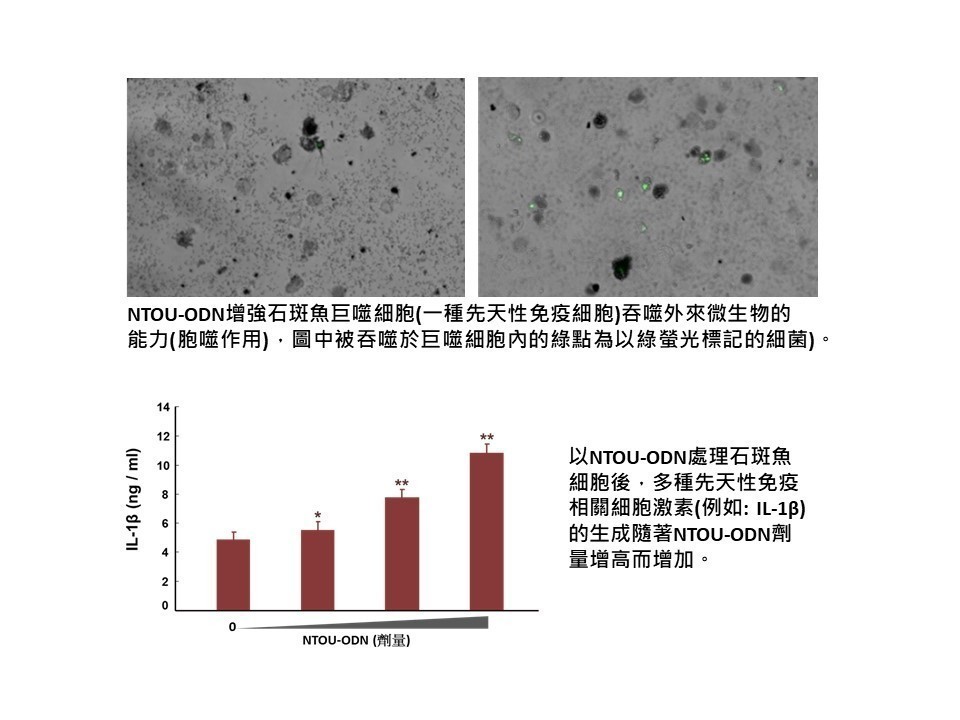
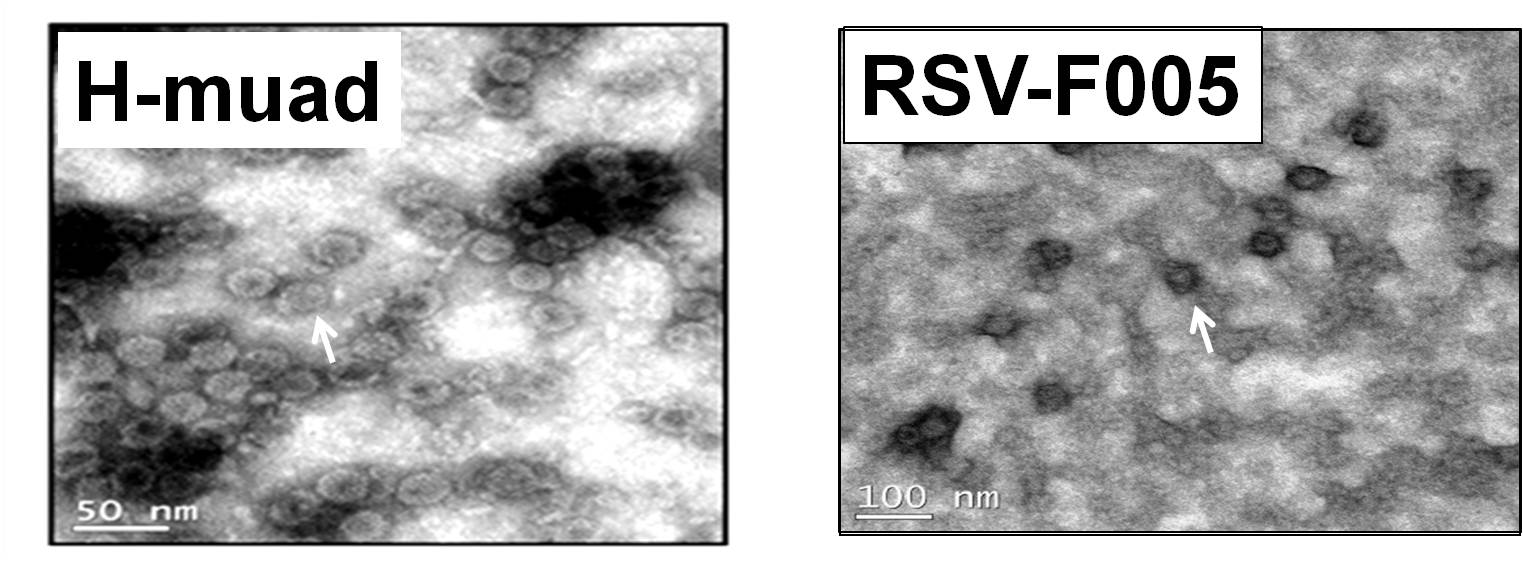
A major challenge in the development of nasal spray vaccines is the use of effective adjuvant. We explored the adjuvant activity of CpG-2722 and cyclic dinucleotide combinations and observed superior effects when the nasally administrated SARS-CoV-2, H7 influenza, and herpes zoster vaccines were adjuvanted with the combinations. Expanded targeted cell populations, enhanced germinal center B cell responses, and reshaped T helper responses are the molecular basis for the enhanced adjuvant effects. |
| Industrial Applicability |
The nasal spray vaccine is a needle-free vaccine. Because it has a variety of characteristics that traditional needle injection vaccines do not have, it has considerable potential in industrial applications. Adjuvant technology plays a key role in the industrial development of nasal spray vaccines. The industrial applicability of the novel nucleic acid structure immune activation composition developed in this study was affirmed in several of our nasal spray infectious disease vaccine studies. |
| Keyword |
Immunostimulant with nucleic acid related structur Toll-like receptor 9 (TLR9) Stimulator of interferon gene (STING) Nasal spray vaccine vaccine adjuvants SARS-CoV-2 Influenza Singles Needle-free vaccine Cancer vaccine |