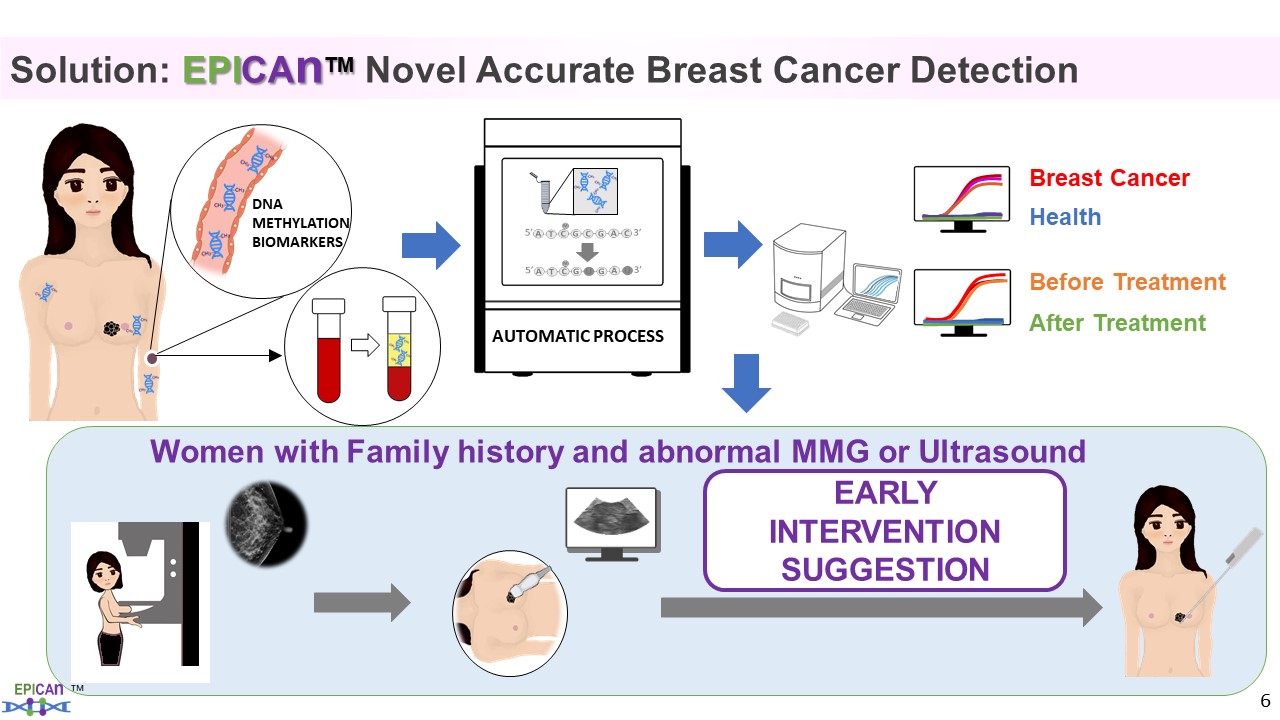
| Summary |
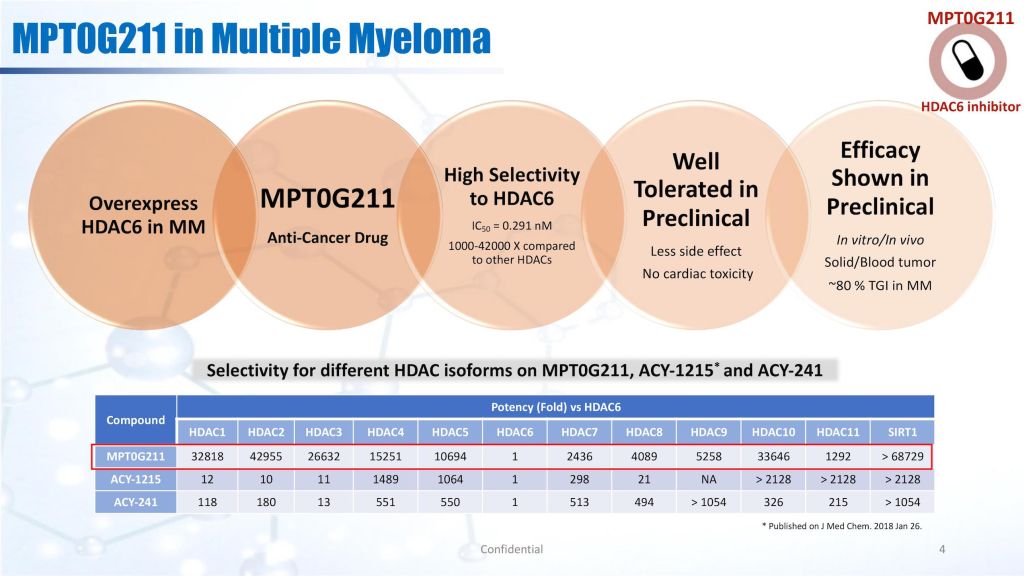
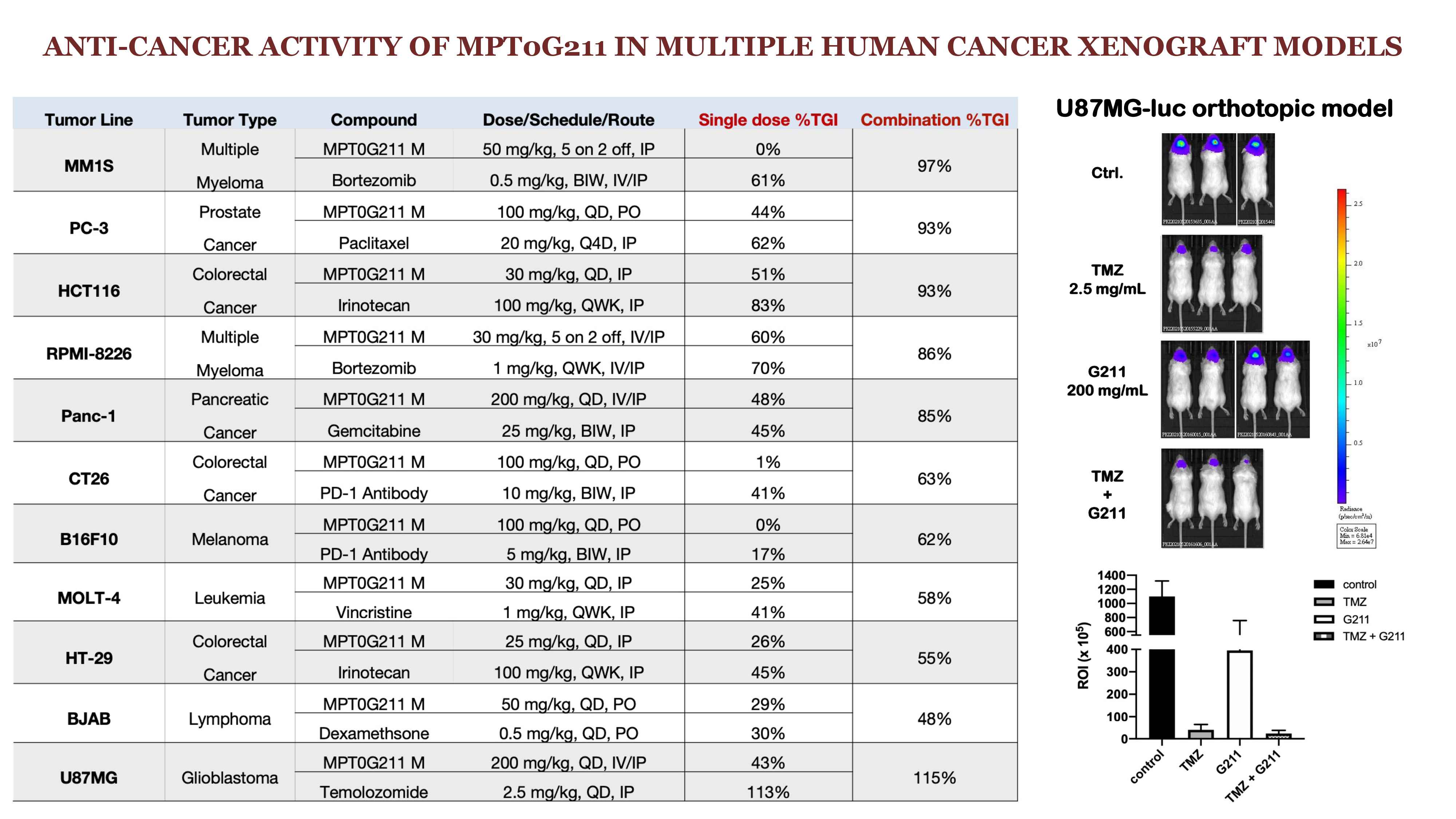
MPT0G211 is a highly selective HDAC6 inhibitor, with comprehensive IP protection. MPT0G211 is mainly focused on multiple myelomabrain tumor indications. For IND package, we have completed CMC in drug substance, CMC in drug product, ADME,most preclinical toxicology studies, only the 28-days repeated dose toxicology study in dog is left. Once the 28-day repeated dose toxicology study in dog is completed, the IND package will be filed in the United StatesTaiwan in Q2 2022.
|
| Scientific Breakthrough |
MPT0G211 has high selectivitysensitivity against HDAC6,it is superior to current competitors (Ricolinostat, ACY1215 Citarionostat, ACY241 developed by Celgene). MPT0G211 can significantly inhibit the survival of circulating tumor cells (CTC) derived from patients with recurrence lung cancerlate stage brain tumor, showing that MPT0G211 has the potential to treat patients suffering from drug resistancerecurrence. MPT0G211 could potentially enter the orphan drug designation to accelerate its development.
|
| Industrial Applicability |
HDAC6 inhibitors are mainly developedfocused on cancer indications by international pharmaceutical companies. The highly selective HDAC6 inhibitor, MPT0G211, has proven to have potential for drug-resistantrecurrent brain cancers. MPT0G211 has been supported by the next-generation translational treatment project by the National Biotechnology Research Park at Academia Sinica. It is expected to file IND in the United StatesTaiwan in Q2 2022.
|