| Technical Name | POLYMERIC BENZOXAZINE RESIN STRUCTURE WITH ADAMANTANE-CONTAINING MAIN CHAIN | ||
|---|---|---|---|
| Project Operator | National Chung-Shan Insitute of Science and Technology | ||
| Summary | A polymeric benzoxazine resin structure with an adamantane-containing main chain produces materials of low dielectric characteristics when cured. |
||
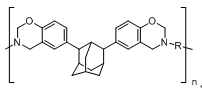
| Scientific Breakthrough | Benzoxazine resin, a member of phenol form aldehyde resins. Benzoxazine resin is characterized in that, when heated, its monomers undergo a ring-opening reaction to cure, and the curing process occurs without producing water or being accompanied by any other side reaction; hence, benzoxazine resin exhibits better workability than the other phenol formaldehyde resins. When film-coated and cured at high temperature, the polymeric benzoxazine resin surpasses non-polymeric benzoxazine in mechanical strength and toughness as well as has a glass transition temperature Tg of 238-260°C. because its main chain displays a high crosslinking density. Hence, the brittleness of polybenzoxazine polymers is alleviated. The benzoxazine synthesized from conventional aromatic diphenol monomers has a dielectric constant Dk of 3.49; the benzoxazine synthesized from aliphatic bisphenol has a dielectric constant Dk of 3.22; and, with adamantane having a rigid structure, introduction of an adamantane structure containing bisphenol increases the glass transition temperature of its cured substance and reduces its dielectric constant Dk to 3.06, thereby indicating that the introduction of an adamantane structure enhances the mechanical properties and glass transition temperature Tg of its cured substance and reduces its dielectric constant. |
||
| Industrial Applicability | 近年來電子產品需求遽增,對於電路板材與封裝材料性質要求日益嚴苛,欲達高性能電子要求,本發明藉由製備主鏈高分子型氧代氮代苯并環己烷(PBZ),提升固化物機械性質與熱性質,更賦予材料優異的撓曲性質,大幅提升其應用性,並且藉由分子鏈段中導入金剛烷(Adamantane) 脂肪族結構,此環狀結構形成立體障礙,增加高分子鏈的剛性,且具有良好的熱穩定性,有效降低固化物介電常數,亦補強固化物之機械性質與強度,預期得到高性能低介電板材,並在印刷電路板材與電子封裝材料應用層面產生重大突破。 |
||
other people also saw