| Technical Name | Nano-chip based drug screening and afterward new drug commercialization | ||
|---|---|---|---|
| Project Operator | National Applied Research Laboratories | ||
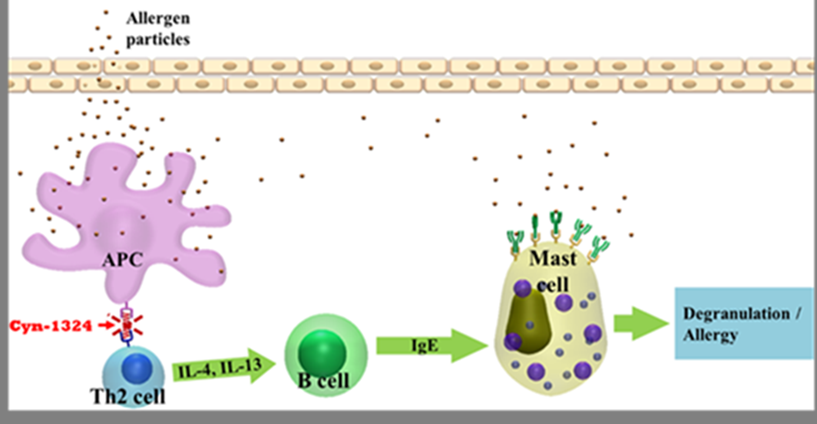
| Summary | We use an innovative chip-based drug screening technology to find a small new molecule, Cyn-1324 that has been experimentally confirmed to have low toxicity and high efficacy for treating with immuno-suppressive diseases such as asthma. The application of IND (investigational new drug) is under preparation. This drug screening method therefore has been identified as for its usefulness and afterward valuable new drug commercialization. |
||
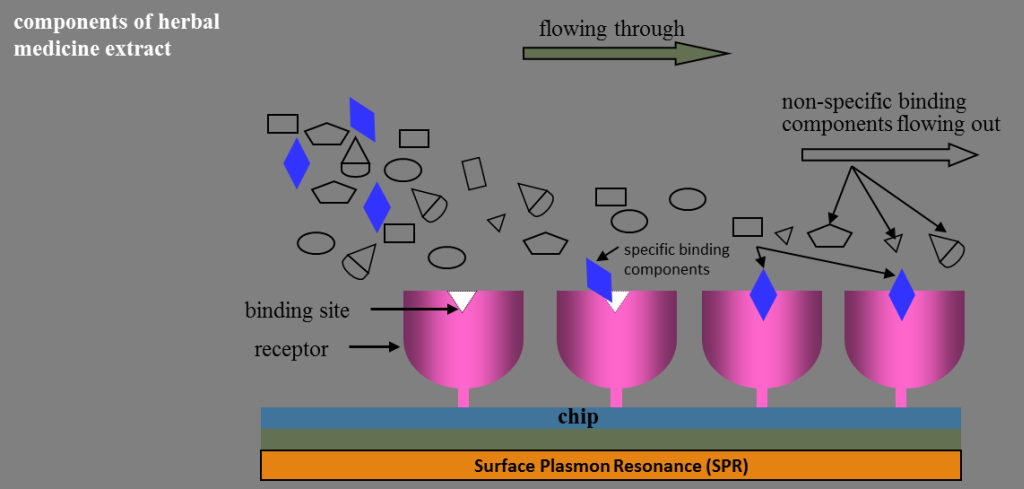
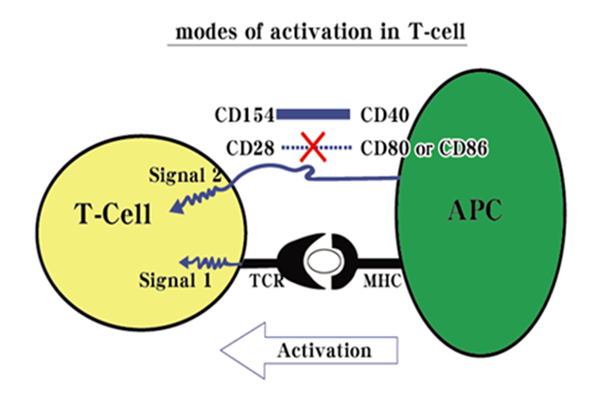
| Scientific Breakthrough | We created an innovative chip-based drug screening technology (two ROC patents granted; one US patent in pending) to find new drug(s) for treating with disease(s). For the first time, we immobilize a T-cell membrane receptor (CD28) on the chip under the influence of an external electric field that can perform an efficient “array” assemble of protein on chip. Consequently, a small molecule (an inhibitor, Cyn-1324; ROC, USA and PROC patents granted) was found that can block the binding between CD28 (T-cell) and CD80 (APC cell) efficiently. Up to now, an IND application (apply for Phase I clinical trial) is under preparation. This chip-based drug screening method is therefore considered as for its usefulness. Many disease-related new drug finding(s) can be done and continued to be developed as for its final goal-commercialization. Both market and industrialization are huge and practical. |
||
| Industrial Applicability | 本篩選技術已包含四個重大疾病領域的新藥篩選與開發: 1. 抗過敏 (如本申請案的抗氣喘新藥,Cyn-1324); 2. 癌症; 3. 糖尿病; 4.骨質疏鬆。 |
||
other people also saw