| Summary |
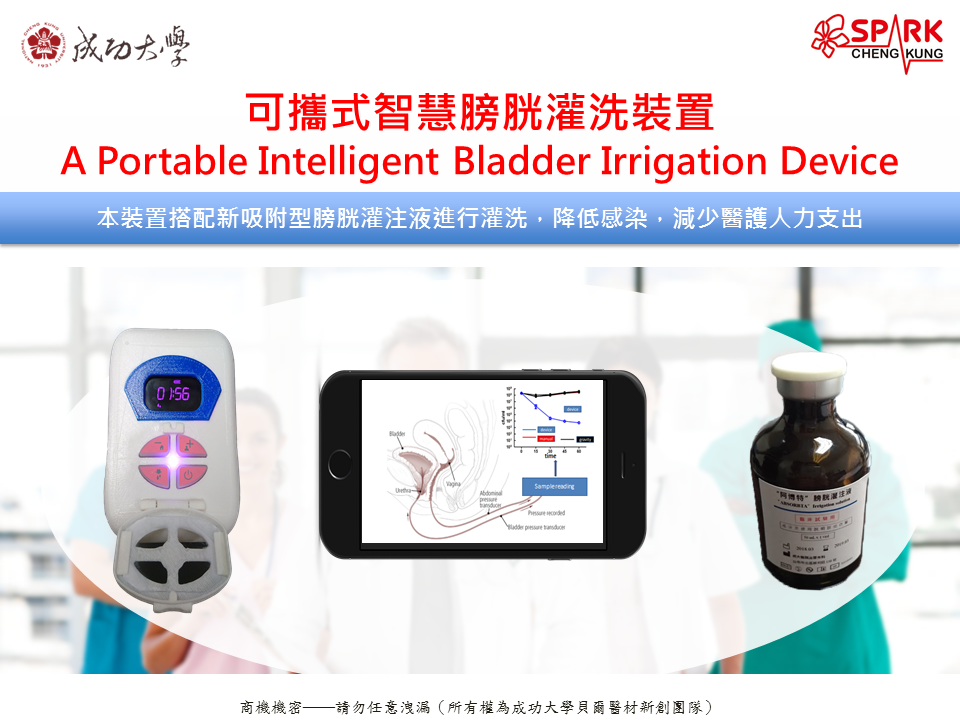
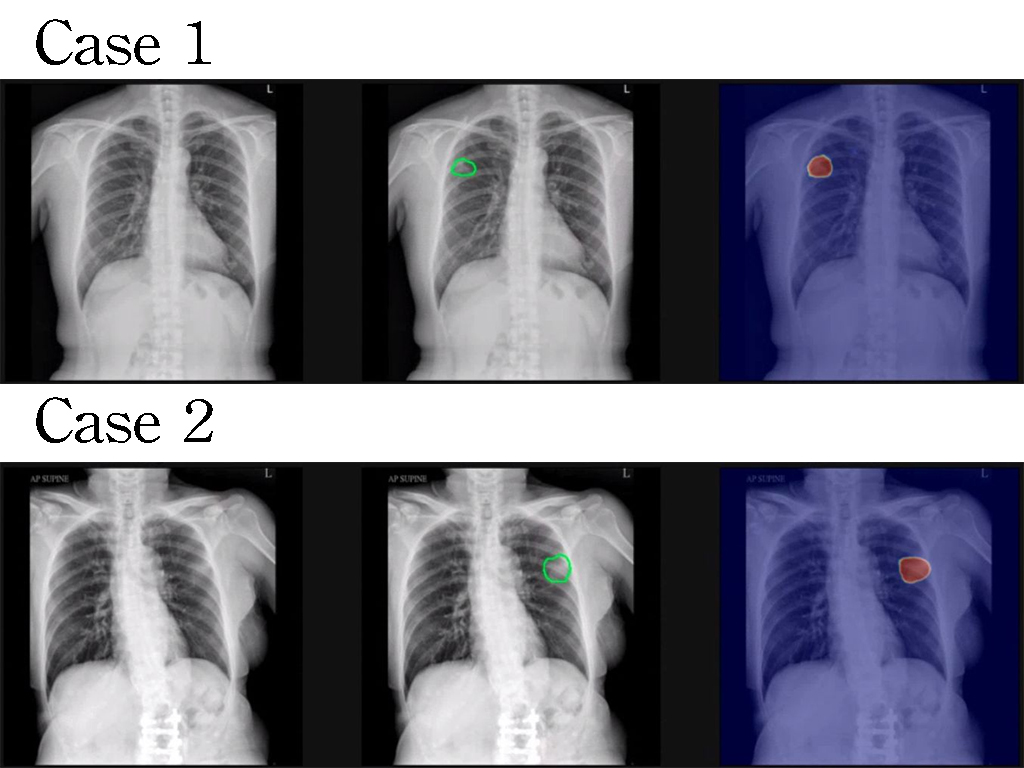
The biofilm formed by bacteria is the main cause of Catheter-Associated Urinary Tract Infections (CAUTIs). Currently, there is a lack of safe and effective strategies for biofilm removal in clinical settings. Our invention, ERAfilm, can remove nearly 95% of the biofilm on catheters removed from CAUTI patients within 10 minutes. It is non-toxic to human bladder cells and does not induce bacterial resistance. ERAfilm holds promise as an important solution for preventing CAUTIs. |
| Scientific Breakthrough |
Biofilms act as shields for bacteria, blocking attacks from the immune system and antibiotics. ERAfilm, composed of two biofilm-penetrating molecules, efficiently eliminates diverse bacterial biofilms on catheters. Non-toxic to human cells and with no induction of bacterial resistance, ERAfilm surpasses other comparators in antimicrobial spectrum and efficiency. It stands as an innovative and valuable invention for CAUTI prevention. |
| Industrial Applicability |
ERAfilm is an innovative biofilm removal technology, primarily targeting the biofilm on urinary catheters to prevent CAUTIs. Moreover, due to its non-invasive and convenient design, its target application areas include hospitals, clinics, long-term care facilities, and home care services. With the increasing global aging population and an increased need for artificial catheters, the demand and market size for ERAfilm are expected to expand annually. |