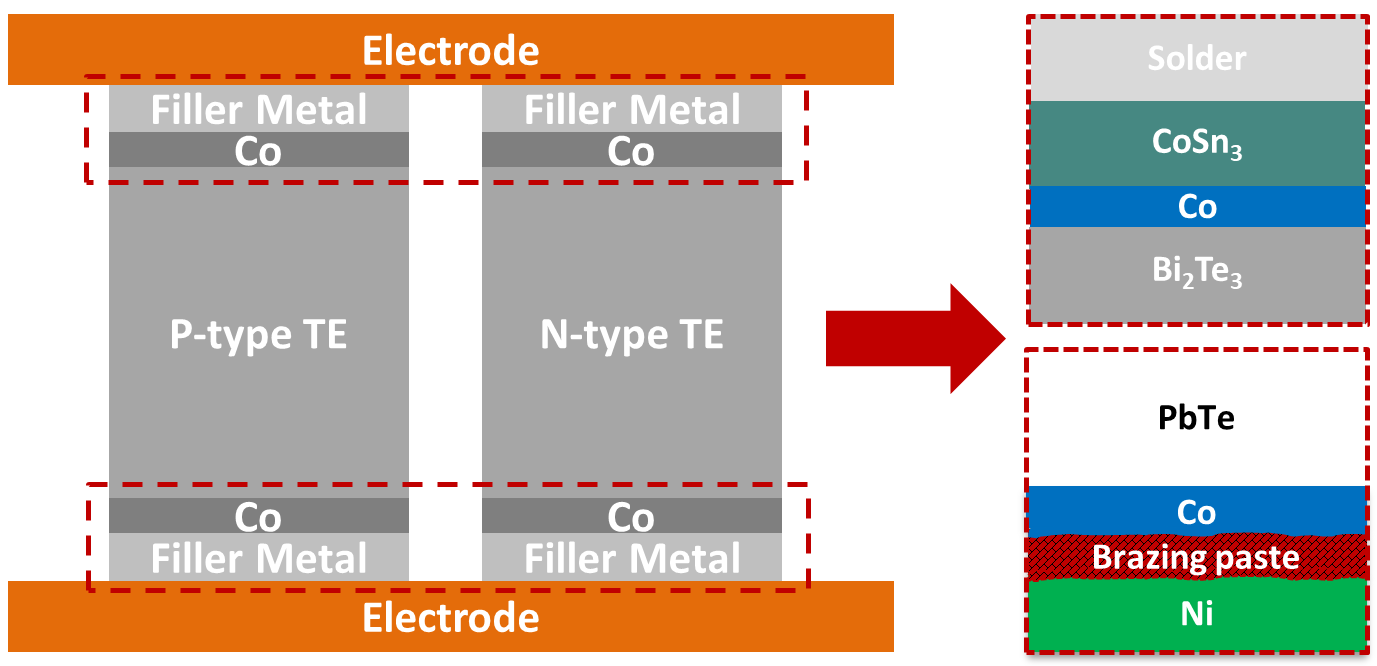
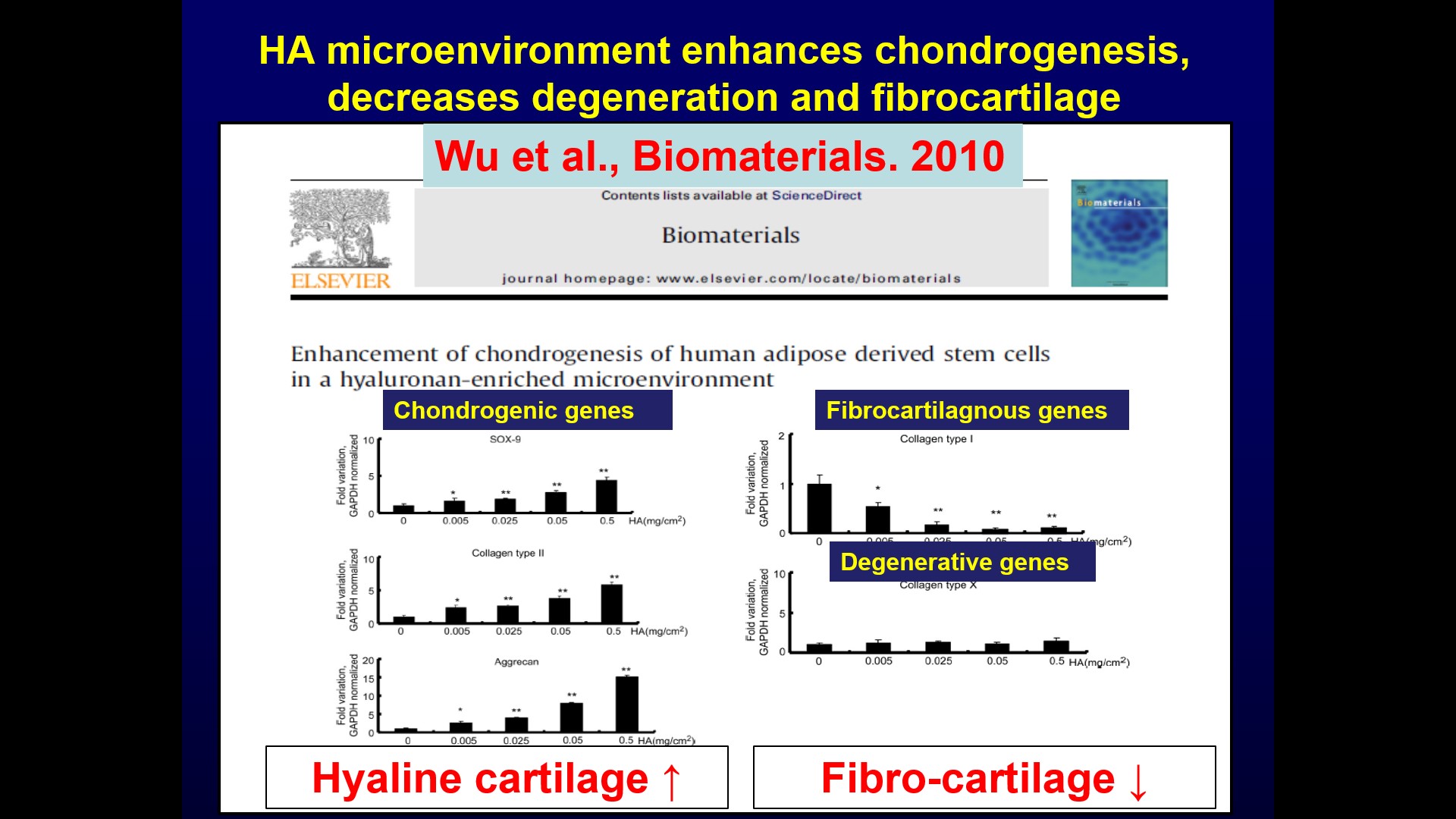
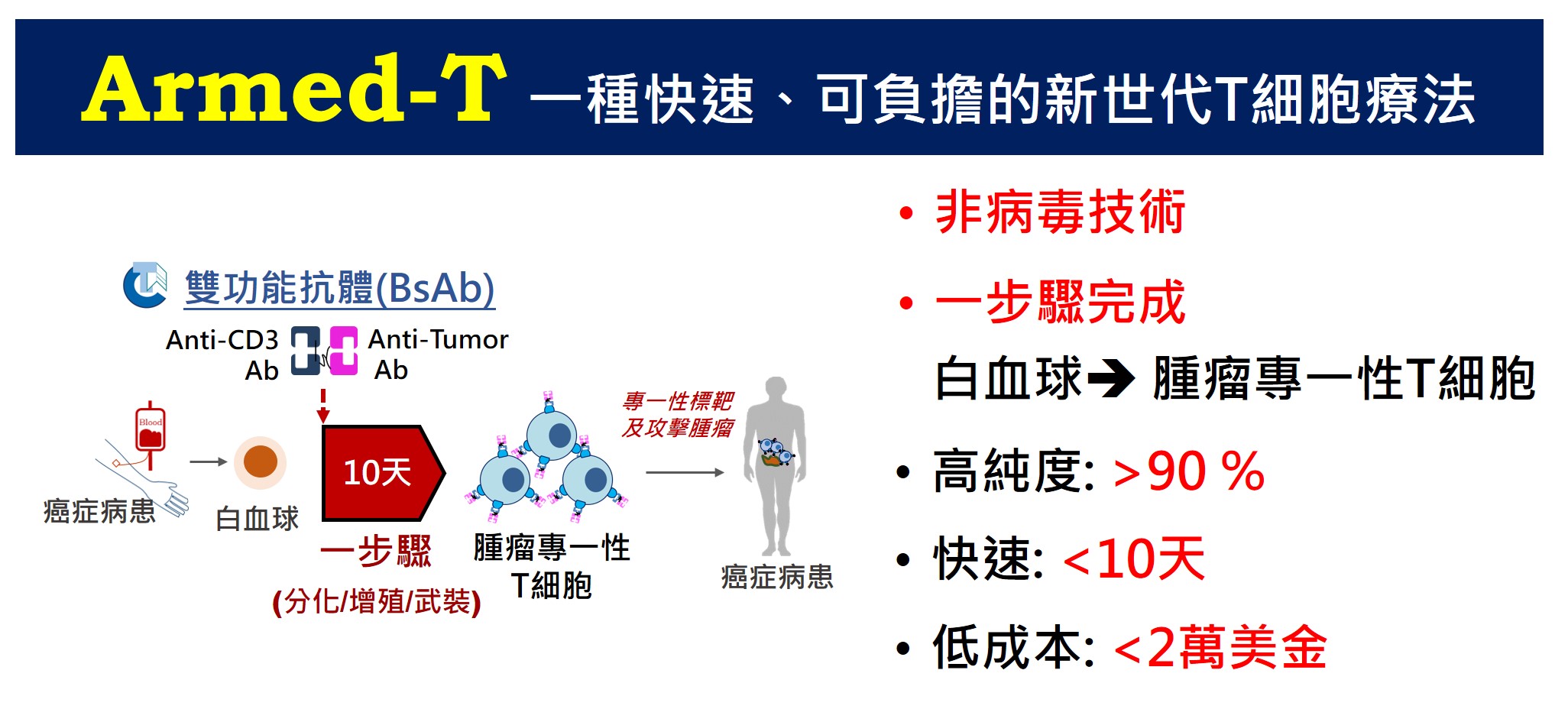
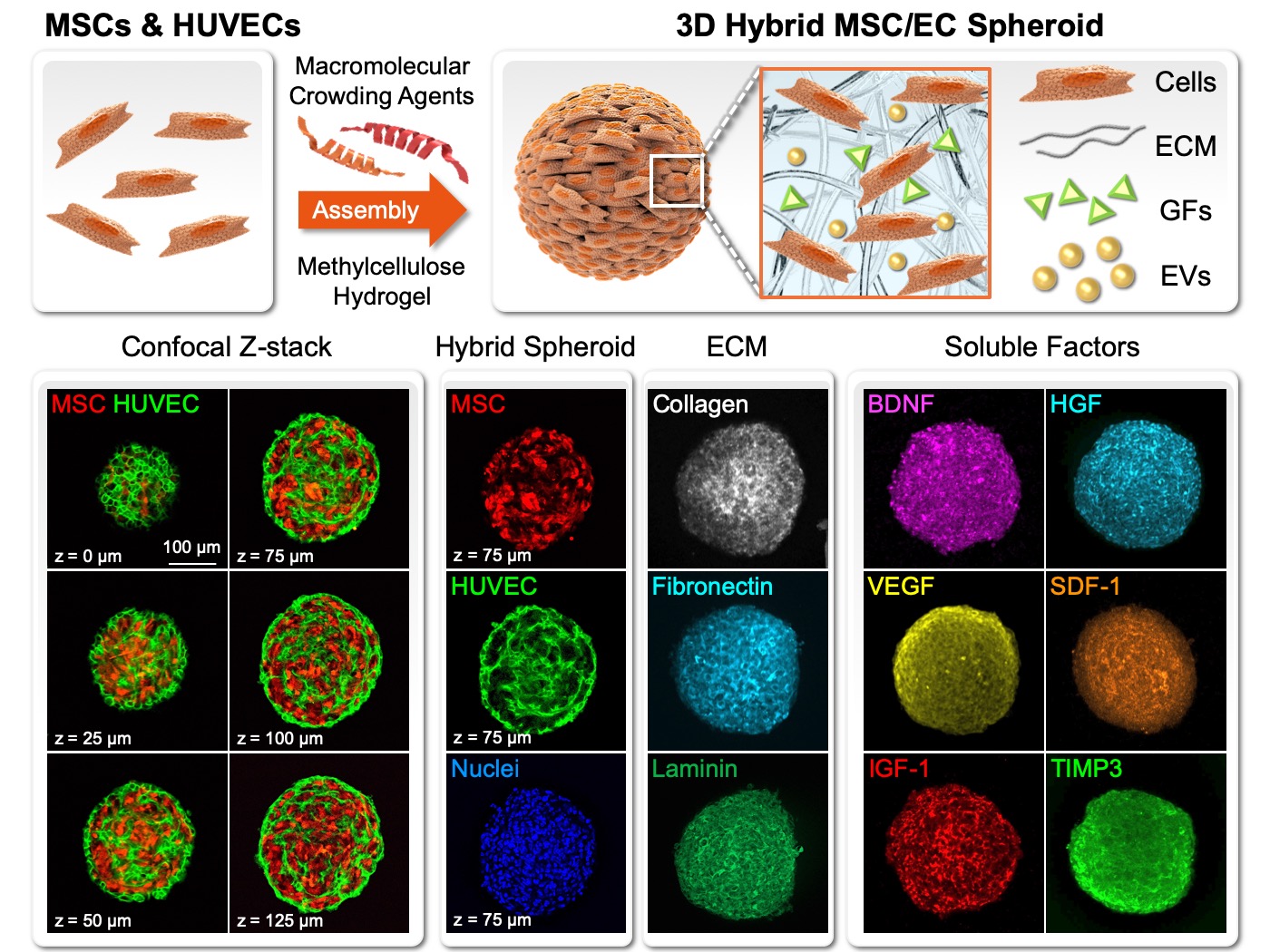
| Technical Name | Systemic Safety Cell Therapy Product Development: Low- Risk in Tumor Promotion for Allogeneic Mesenchymal-stem-cell Products derived from Maternal Placenta (Zi He Che) | ||
|---|---|---|---|
| Project Operator | Taipei Medical University | ||
| Project Host | 林泰元 | ||
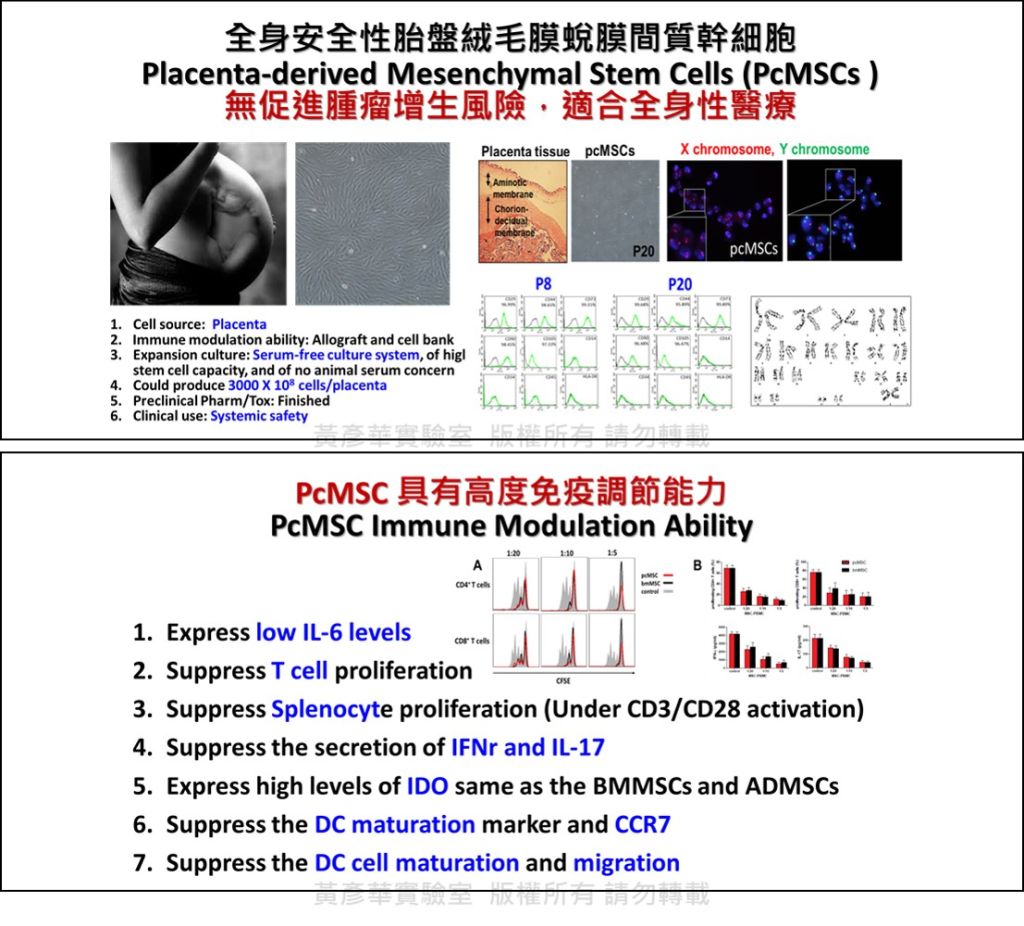
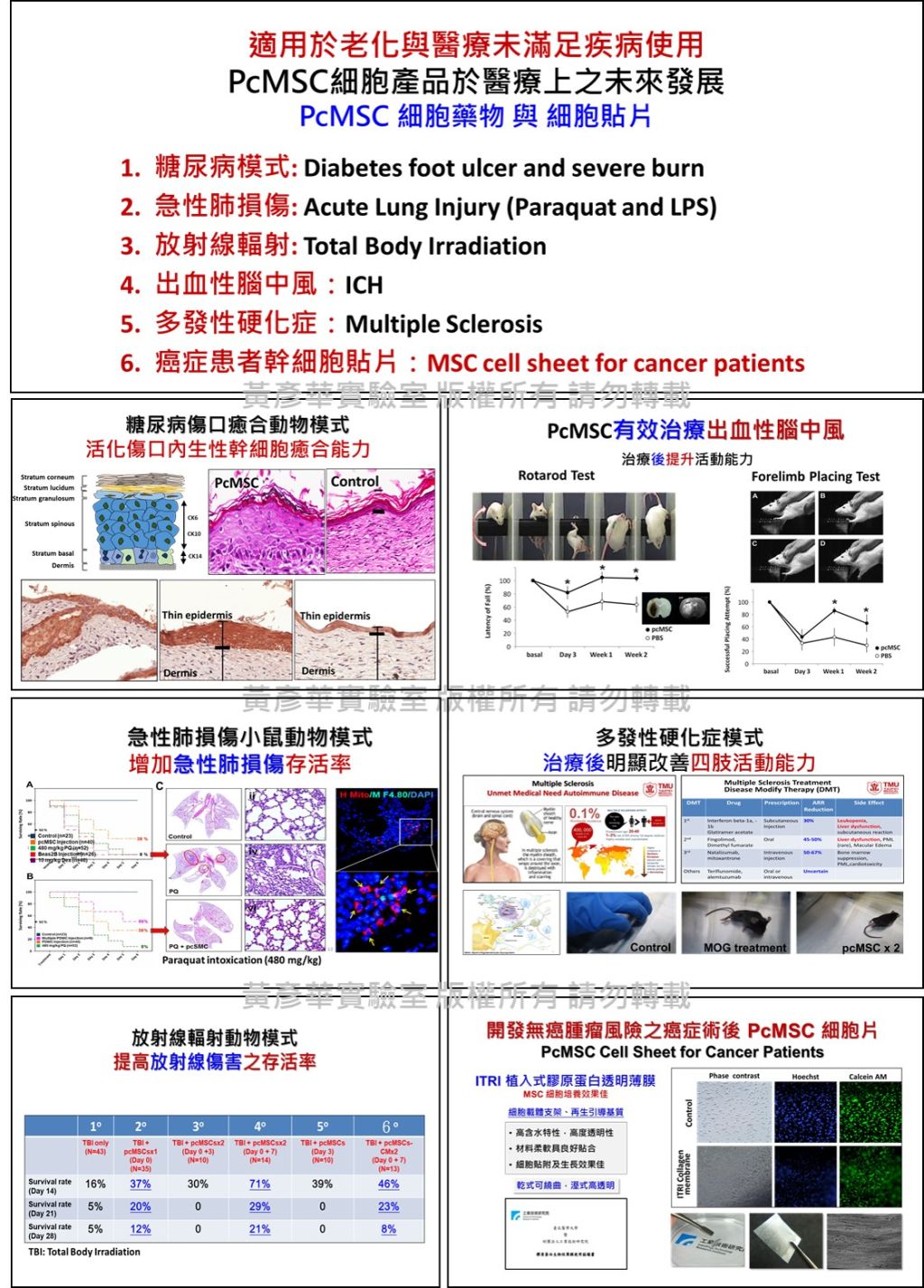
| Summary | The serum-free selection culture method was applied to generate human placenta maternal-part (choriodecidual membrane) mesenchymal stem cells (pcMSCs), which could be used to cure acute severe injury; pcMSCS could be sub-cultured more than 20-passages thus could make a cell-bank for emergency use; importantly, pcMSCs could not promote tumor growth and sometime could inhibits certain tumors growth. |
||
| Scientific Breakthrough | 1.Serum-free selection culture method (2 patents in Taiwan). |
||
| Industrial Applicability | We have successfully generated the clinical grade human pcMSCs with high passage capacity (>20 passages) using novel serum-free selection cultivation. The pcMSC unique characters in stemness stability and free of tumor growth risk highly benefit the future patient affordable clinical allogenic cell therapy, in particular for the systemically safe use of age-related diseases and cancer patients. |
||
| Keyword | Human placenta choriodecidual me Serum-free selection culture method Systemic application in safety Severe (including diabetes) wound injury Multiple sclerosis Acute lung injury Acute radiation injury Acute graft-versus-host disease (gvhd) Acute kidney injury Cell-sheet | ||
- rita1204@tmu.edu.tw
other people also saw