| Technical Name | Drug Recycle: Recovery of Active Pharmaceutical Ingredients from Various Medications by Solvent Extraction and Recrystallization | ||
|---|---|---|---|
| Project Operator | National Central University | ||
| Project Host | 李度 | ||
| Summary | Drug recycle process was devised to prevent active pharmaceutical ingredients (APIs) from polluting the soil and water bodies. Treating waste drug tablets by extraction-recrystallization-rinsing, API from the tablets can be recovered with high yield and purity. In addition, the tablet residue as leftover was successfully converted into bioplastic using trifluoroacetic acid treatment. |
||
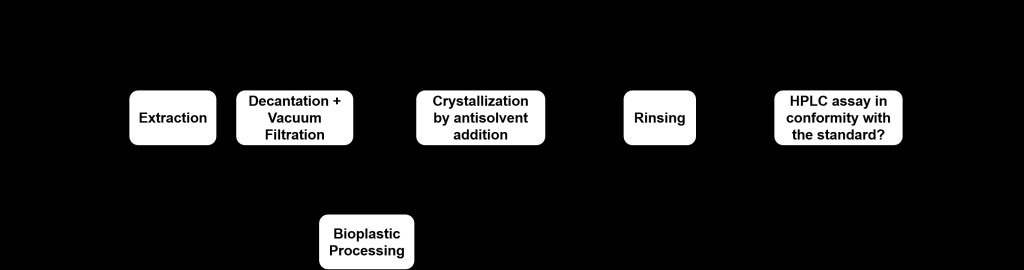
| Scientific Breakthrough | The commercial ibuprofen tablets from different manufacturers were recycled. The yield of the recycle process was 76% and the assay was 99.7%, which was in conformity with US Pharmacopeia standard. No impurities were detected by 1H- and 13C-NMR spectroscopy. Moreover, the tablet residue generated from the recycling process was successfully converted into bioplastic with acceptable toughness. |
||
| Industrial Applicability | Our recycle process consists of extraction, crystallization, and rinsing, which are common industrial unit operations. Only stirred-tank vessel, filtration, and drying equipment are required. The recycling process can be performed for drug products from different manufacturers, as long as the API contained is the same. Because of its simplicity and robustness, scale-up should be straightforward. |
||
| Keyword | Recycle drug product, green technology waste treatment active pharmaceutical ingredient excipient extraction crystallization separation technique bioplastic | ||
- xp871115@gmail.com
other people also saw