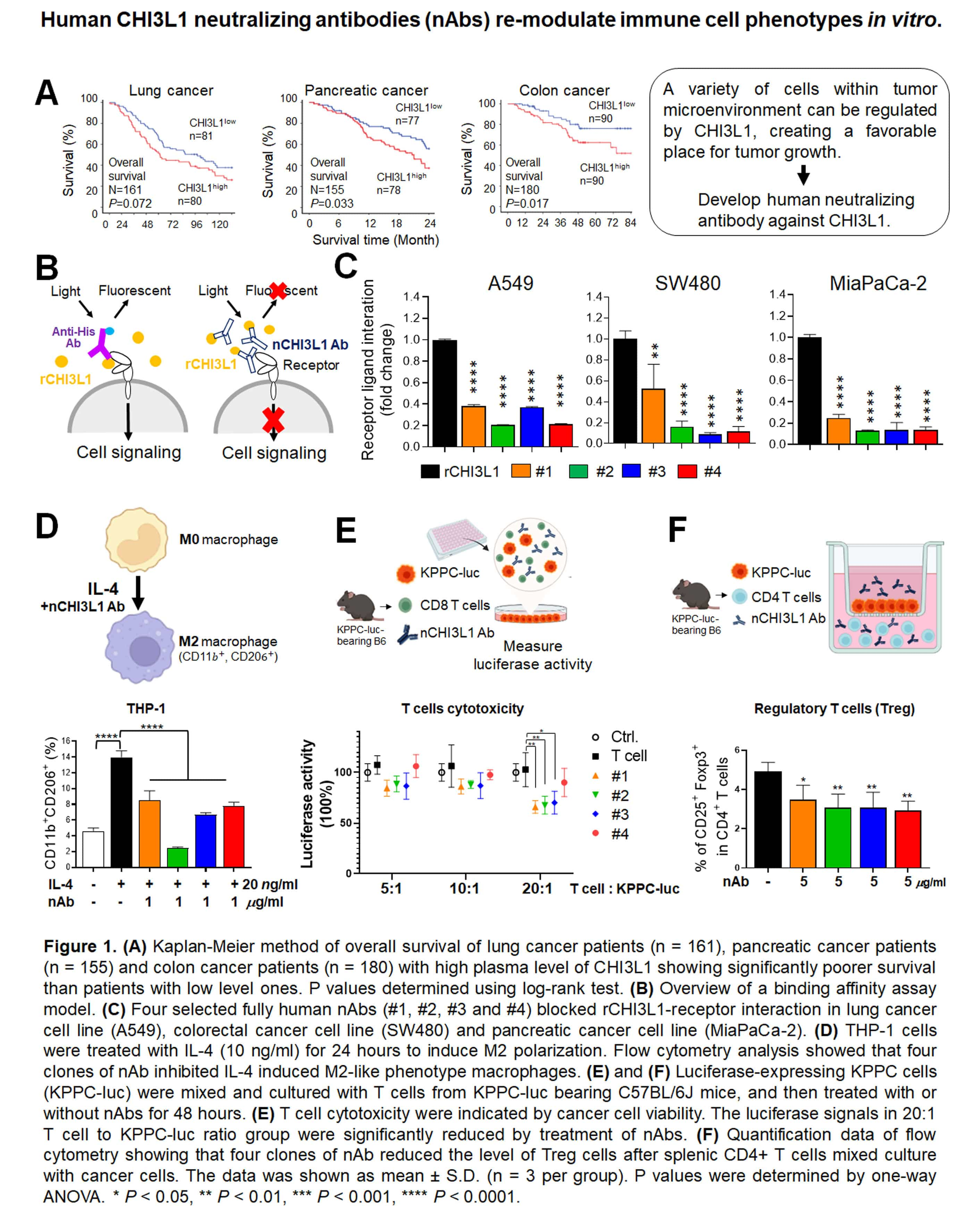
| Technical Name | Next-Generation Immune- and Anti-Angiogenetic Therapy against Hepatocellular Cacinoma: Combination Therapy of CXCR4 Antagonist DBPR807 and PD-1 Antibody or Kinase Inhibitor Sorafanib | ||
|---|---|---|---|
| Project Operator | National Health Research Institutes | ||
| Project Host | 夏克山 | ||
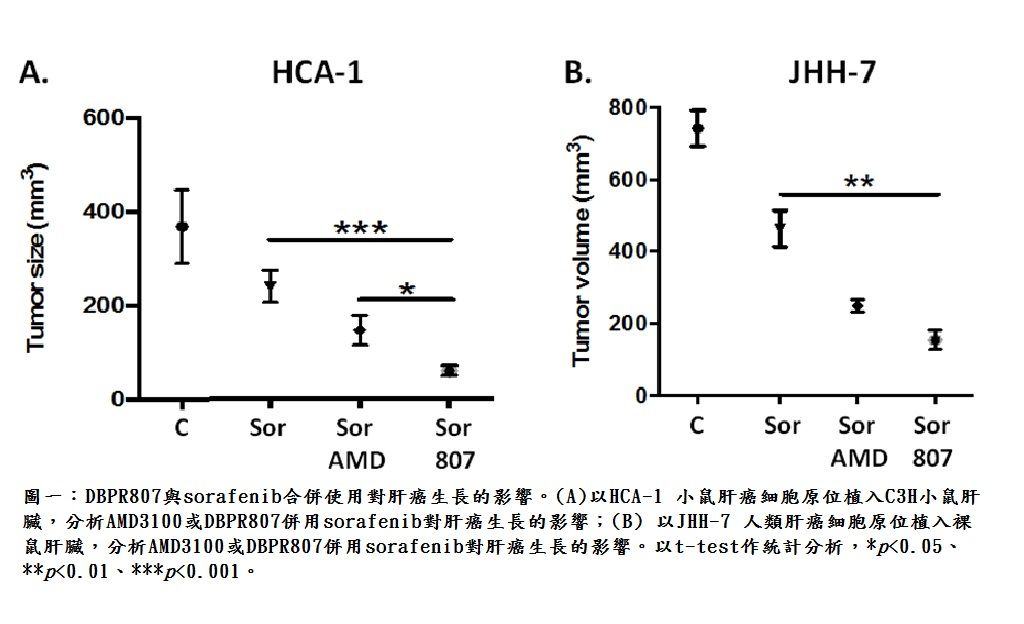
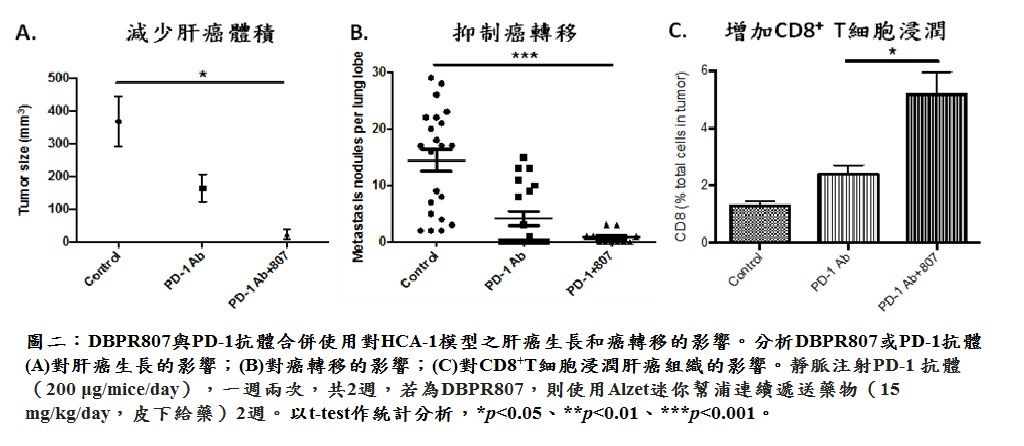
| Summary | The proof-of-concept of DBPR807 on HCC disease animal models revealed that as it was combined with sorafenib (tumor size, -80%), a first-line marketed multikinase inhibitor or PD-1 antibody (tumor size, -95%), an immune checkpoint inhibitor, the tumor growth and cancer metastasis could be dramatically inhibited as compared to the single treatment with sorafenib (-33%) or PD-1 antibody (-53%). |
||
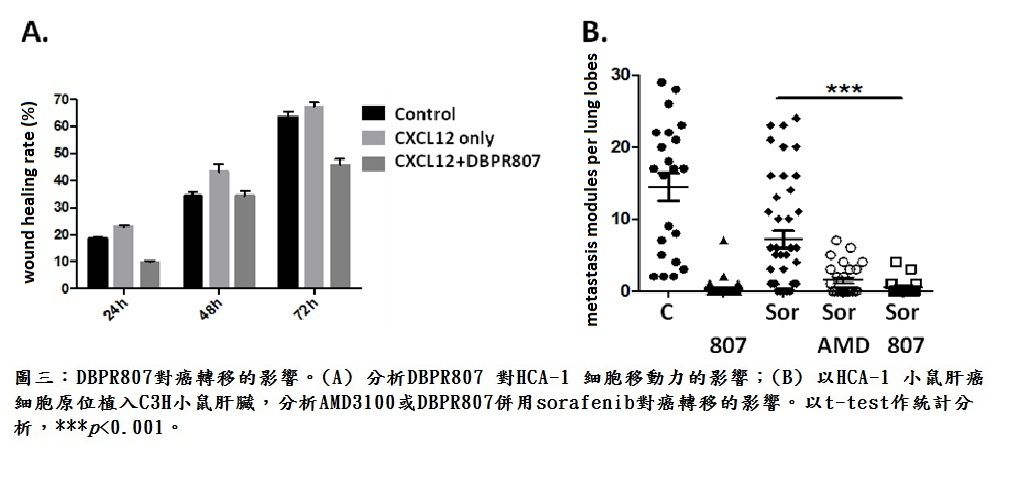
| Scientific Breakthrough | Combination therapy of DBPR807 with sorafenib or PD-1 Ab has been shown to have better efficacy in disease animal models orthotopically implanted with HCA-1 or JHH-7 liver cancer cells than the single treatment of sorafenib or PD-1 Ab. This novel combination protocol can simultaneously inhibit angiogenesis, enhance CTLs infiltration and hamper cancer metastasis, leading to a new horizon of cancer treatment. |
||
| Industrial Applicability | There are four FDA approved anti-liver cancer drugs on the current market, including sorafenib (USD one billion per year), regorafenib, lenvatinib and nivolumab in which the IP right of sorafenib will expire on 2020/1/12; thus, its combination therapy with DBPR807 against liver tumor is developed. Theoretically, DBPR807 is allowed to combine with any kinase or immune checkpoint inhibitors to treat liver cancer and other highly metastatic cancers. |
||
| Keyword | CXCR4 receptor antagonist combination therapy immunotherapy hepatocellular carcinoma kinase inhibitor immune checkpoint inhibitor nanoparticle formulation metastasis infiltration | ||
- ksshia@nhri.org.tw
other people also saw