| Technical Name | Autoantibody-Targeted Etiological Diagnosis for IgA Nephropathy | ||
|---|---|---|---|
| Project Operator | National Defense Meical Center, Taipei, Taiwan | ||
| Project Host | 賈淑敏教授 | ||
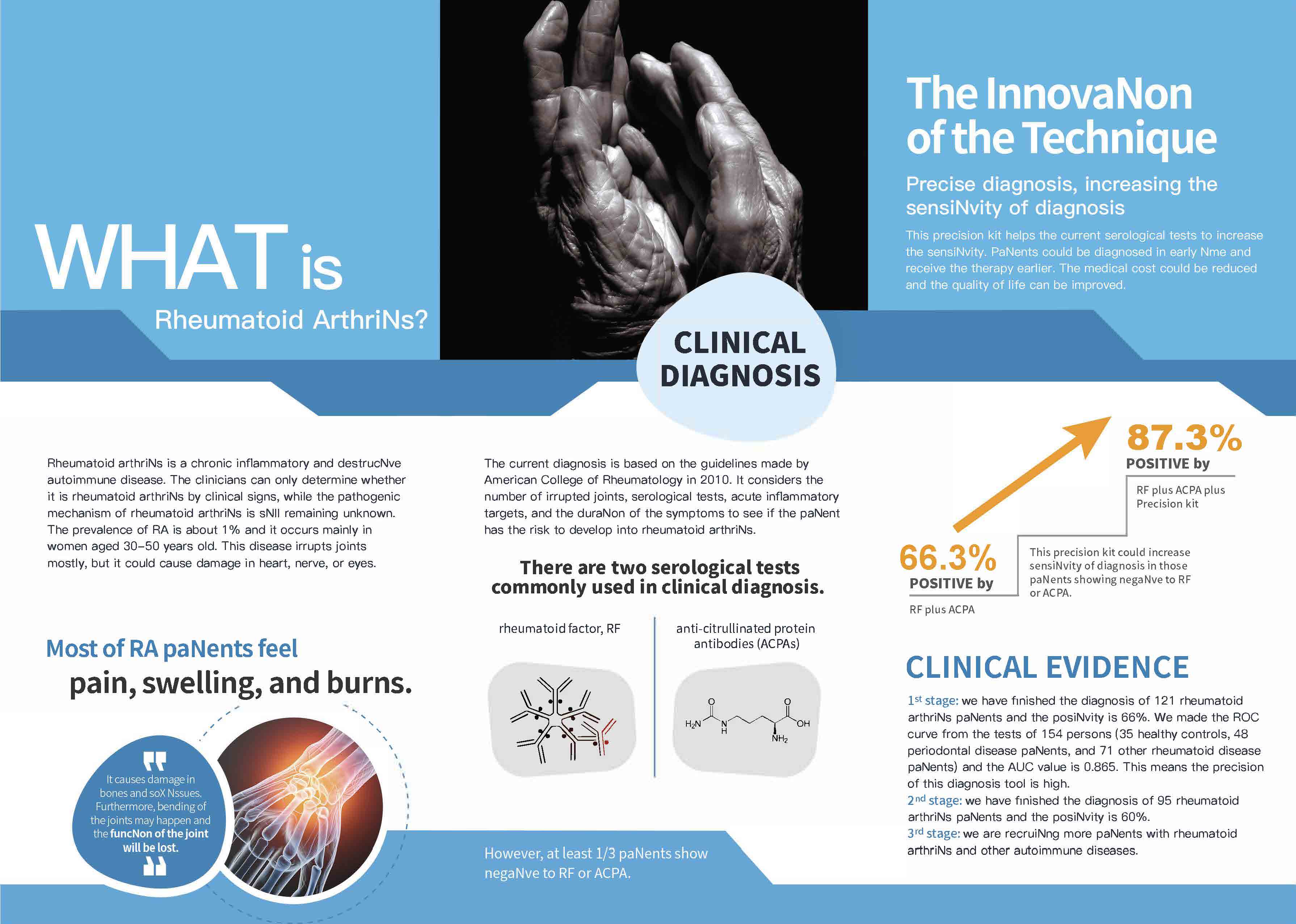
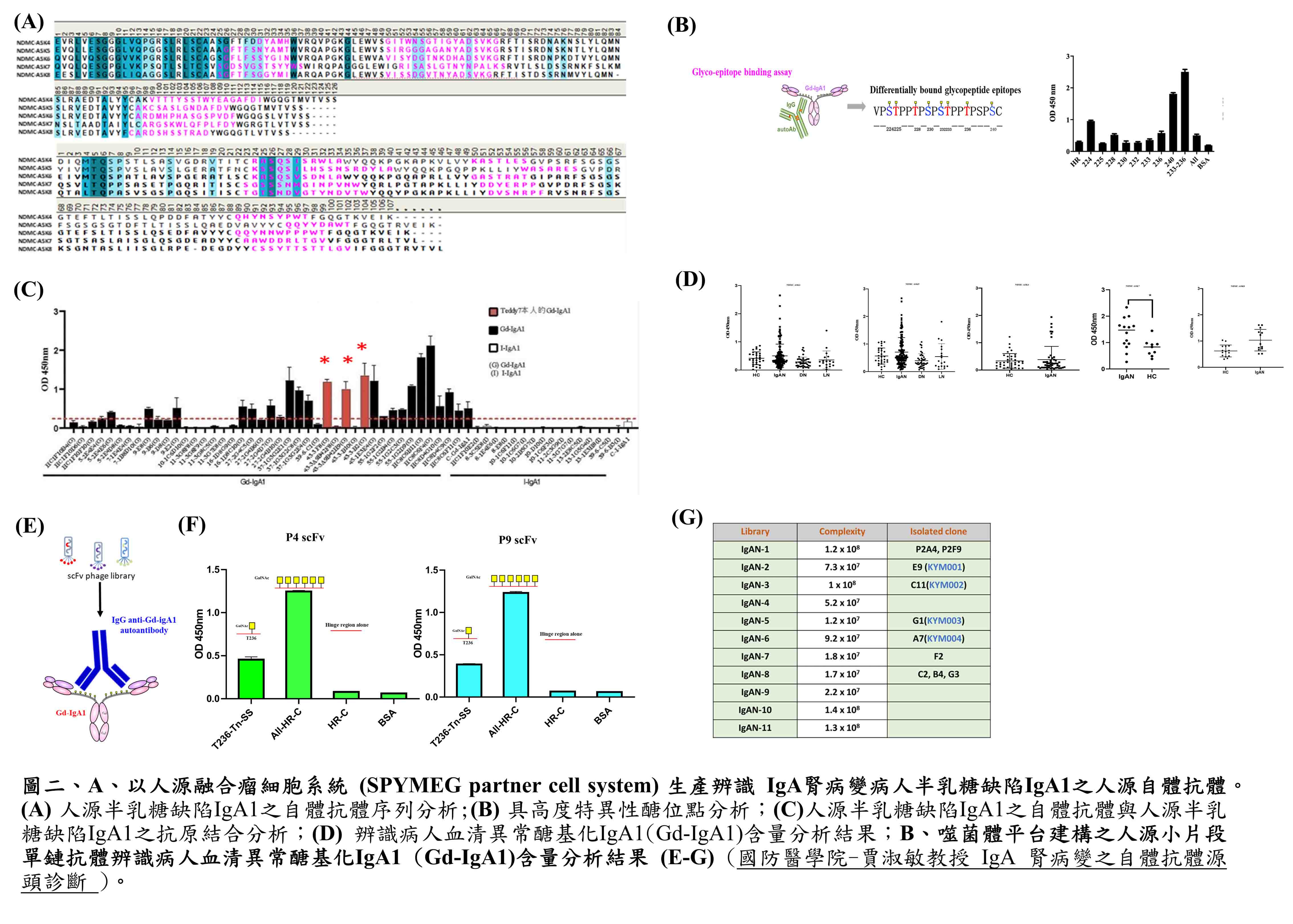
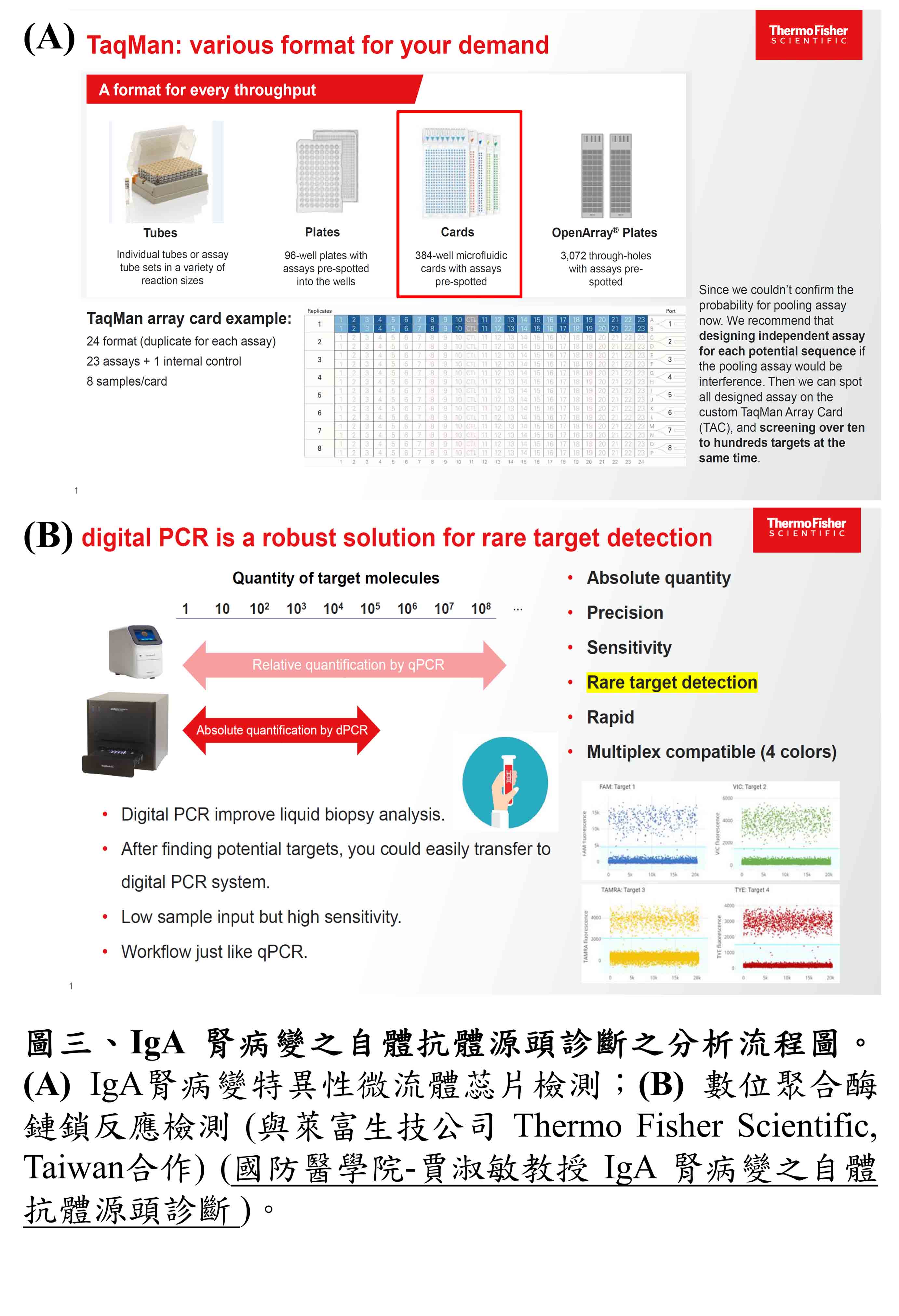
| Summary | Our invention boasts its feasibilityvalue in a non-invasive, etiological diagnosis for IgA nephropathy (IgAN), by: (1) an IgAN specific real-time PCR assay (2) Applied Biosystems TaqMan assay 24-384-well TaqMan Array cards (microfluidic cards)(3) Digital PCR assay. These newly developed knowhow-based steps serve as a well-documented example for most of the other autoimmune diseases in generating etiological, non-invasive diagnostic reagents. |
||
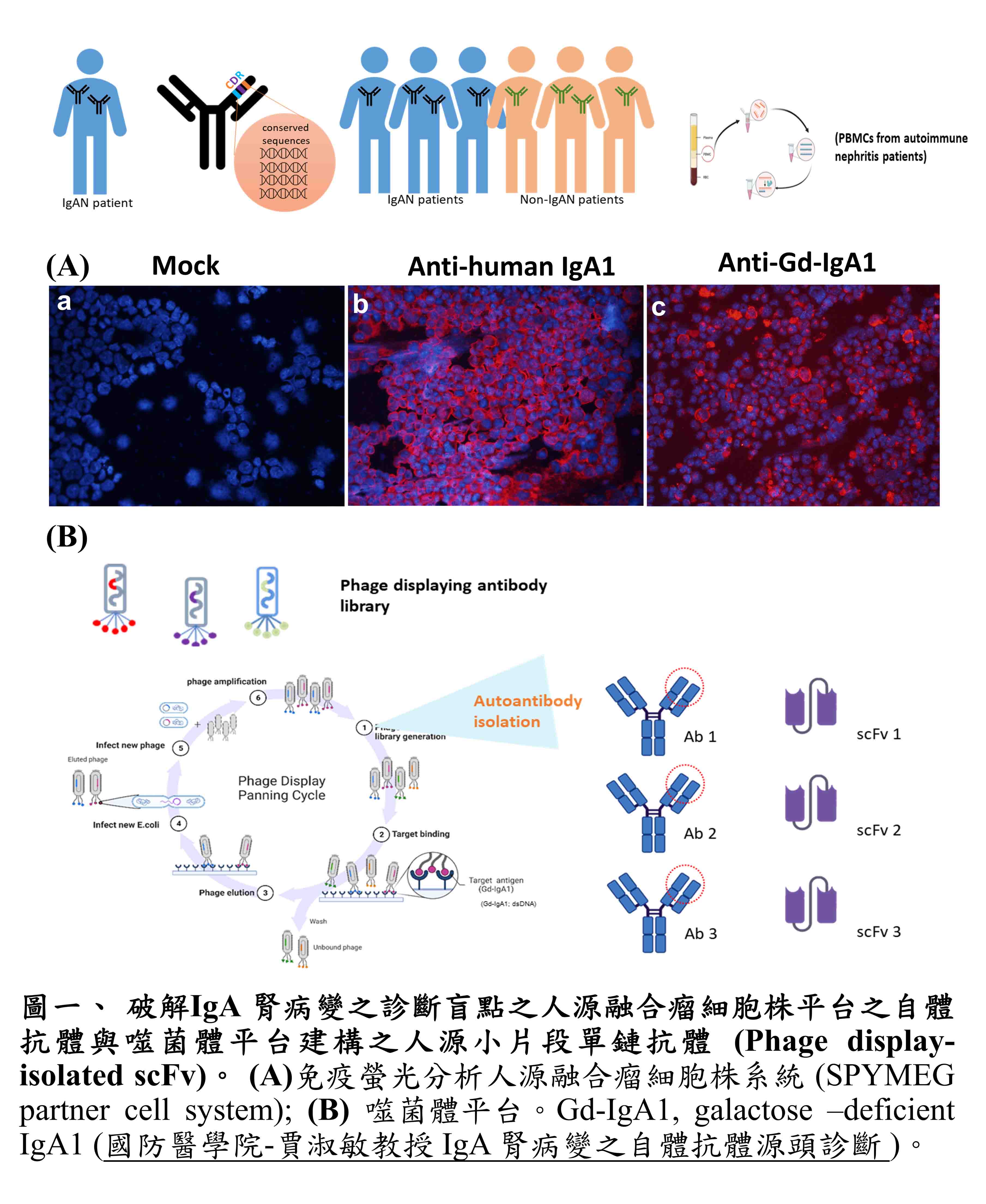
| Scientific Breakthrough | To develop non-invasive measures for etiological, early diagnosis of IgA nephropathy (IgAN) is clinicallyurgently warranted. To do this, we have been successful in demonstrating for the first time a set of IgG autoantibodies against IgA1 autoantigensthereby established the following diagnostic tests: (1) an IgAN specific real-time PCR (2) Applied Biosystems assay 24-384-well TaqMan Array cards (microfluidic cards)(3) Digital PCR assay. |
||
| Industrial Applicability | Our invention with the following tests: (1) an IgAN specific real-time PCR assay (2) Applied Biosystems TaqMan assay 24-384-well TaqMan Array cards (microfluidic cards)(3) Digital PCR assay. These newly developed knowhow-based steps will be able to serve as well-documented examples for most of other autoimmune diseases in especially generating etiological, non-invasive diagnostic reagents. Our estimated benefits as follows: Domestic – NT$460 million Global - NT$20 billion. |
||
| Keyword | IgA nephropathy Human hybridoma Human autoantibody Phage-display system Single-chain variable fragments Etiology Non-invasive Digital PCR TaqMan Array cards with microfluidic cards | ||
- Contact
- Shuk-Man Ka
- shukmanka@gmail.com
other people also saw