| Technical Name | Bispecific Nanobody-Engineered CAR.BiTE-γδT Cell Therapy with Allogeneic Potential | ||
|---|---|---|---|
| Project Operator | Translaltional Cell Therapy Center, China Medical University Hospital | ||
| Project Host | 黃士維 | ||
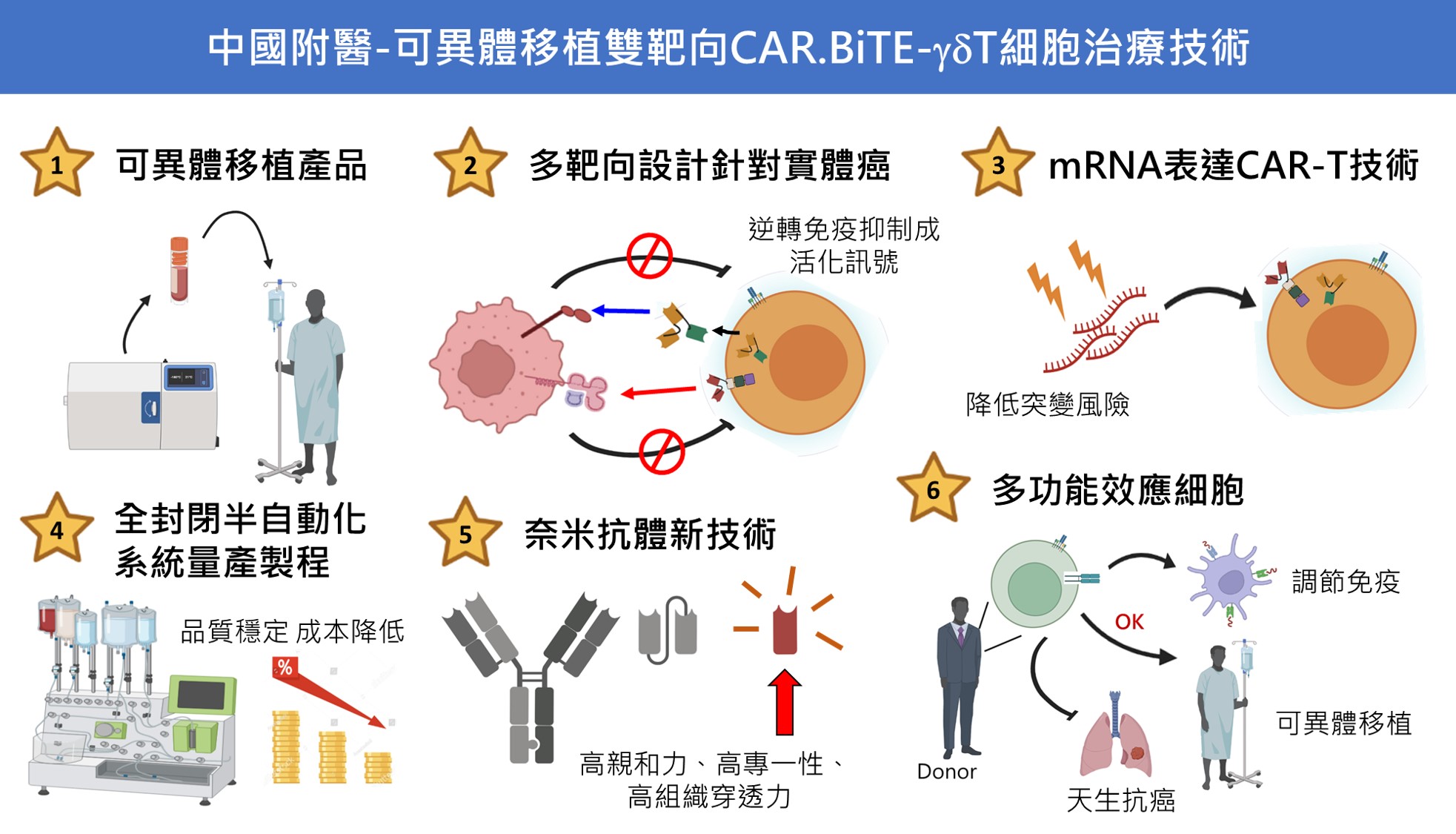
| Summary | 1. Dual targeting design for overcoming immune suppressionto synergize with bystander immune cells to against solid tumors 2. γδT as effector cellsoffer allogeneic potentials 3. mRNA-driven, nanobody-based engineered CAR.BiTE construct 4. Adapted manufacturing by a semi-automated close-system 5. Cell prototype is adherent to the guidance of U.S. FDA for allogeneic applications 6. Low immunogenicity of Nb-based CARBiTE moieties 7. Patents were granted |
||
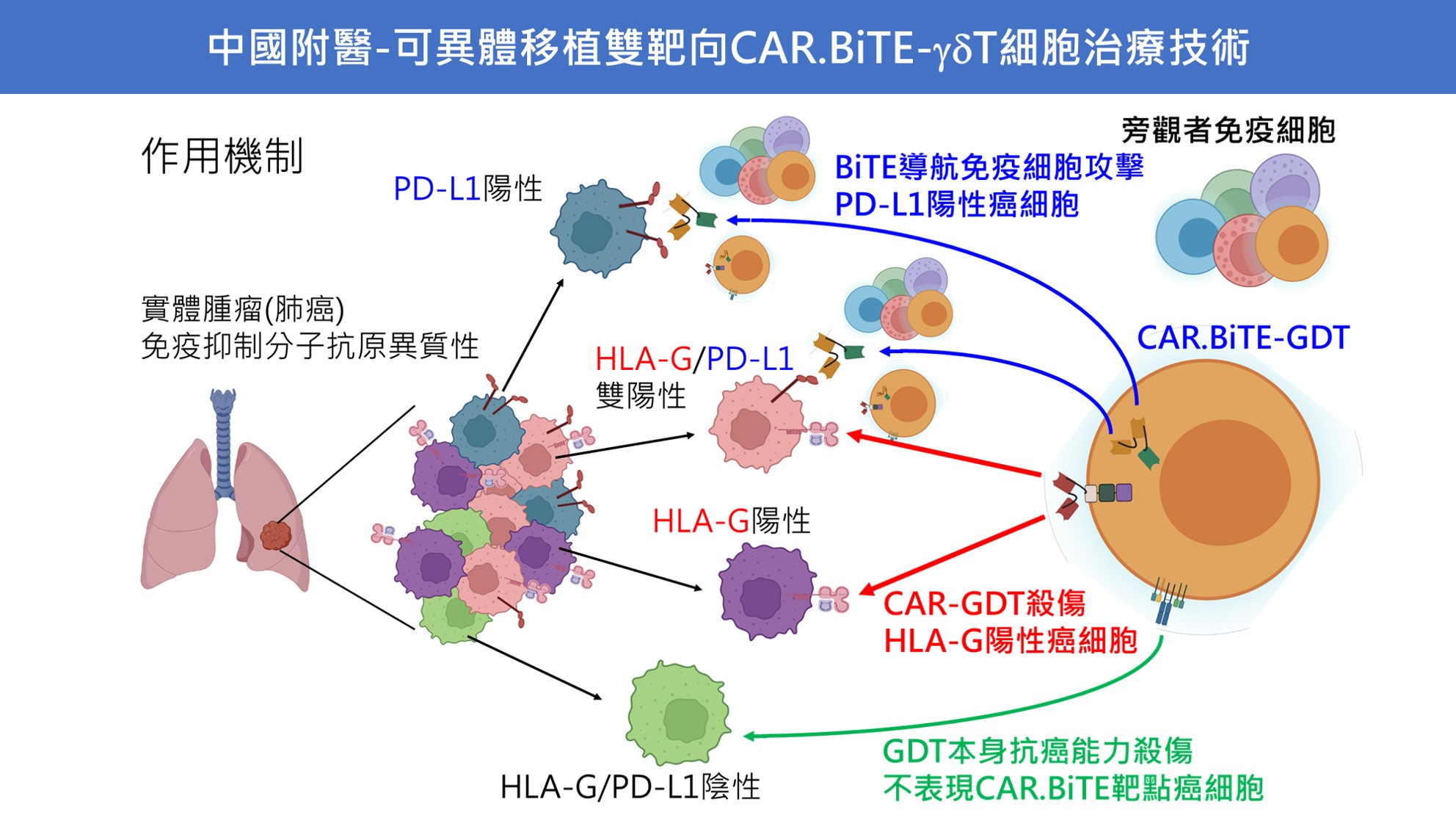
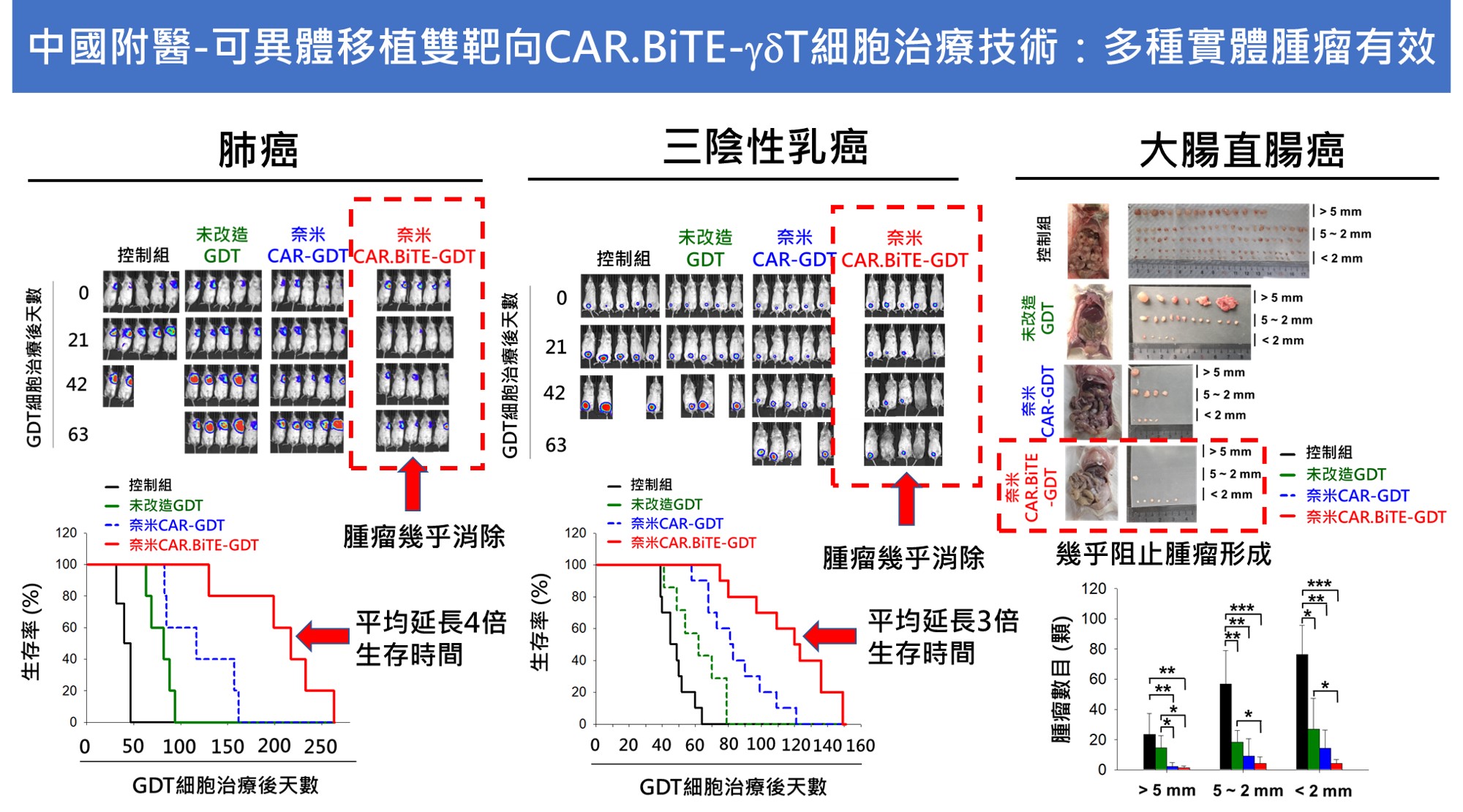
| Scientific Breakthrough | 1. First nanobody-based bivalent CAR.BiTE-γδT cell therapy through electroporation with mRNA2. CAR able to reverse inhibitory tumoral HLA-G to activate immune response, secreted BiTE triggers bystander effectors to against PD-L1+ tumor cells,γδT cells exert its natural antitumor activity to ablate antigen-low tumor cells3. First semi-automatic close-system adapts to cultivate γδT cells from donors4. No obvious toxicity to normal tissues5. Allogeneic potentiallow immunogenicity |
||
| Industrial Applicability | 1. Absolutely I.P. values of nanobodiesCAR.BiTE design by granted patents.2. Novel semi-automatic close-system for manufacturing large scale cell product in one batch to reduce costs.3. Off-the-shelf allogeneic application is more adherent to clinical operations than autologous personal approaches.4. Target indications are solid tumors including lung, breastcolon cancers, that the potential marketing size is considered as 10-fold of current CAR-T products. |
||
| Keyword | Allogeneic solid tumor HLA-G PD-L1 Nanobody chimeric antigen receptor bispecific T cell engager γδT mRNA close system | ||
- Contact
- Shi-Wei Huang
- seso4505@hotmail.com
other people also saw