| Technical Name | Recycling VanadiumProton-Exchange Membranes from Waste Vanadium Flow Batteries through Ion ExchangeRecast Methods | ||
|---|---|---|---|
| Project Operator | National Cheng Kung University Hierarchical Green-Energy Materials (Hi-GEM) Research Center | ||
| Project Host | 陳偉聖 | ||
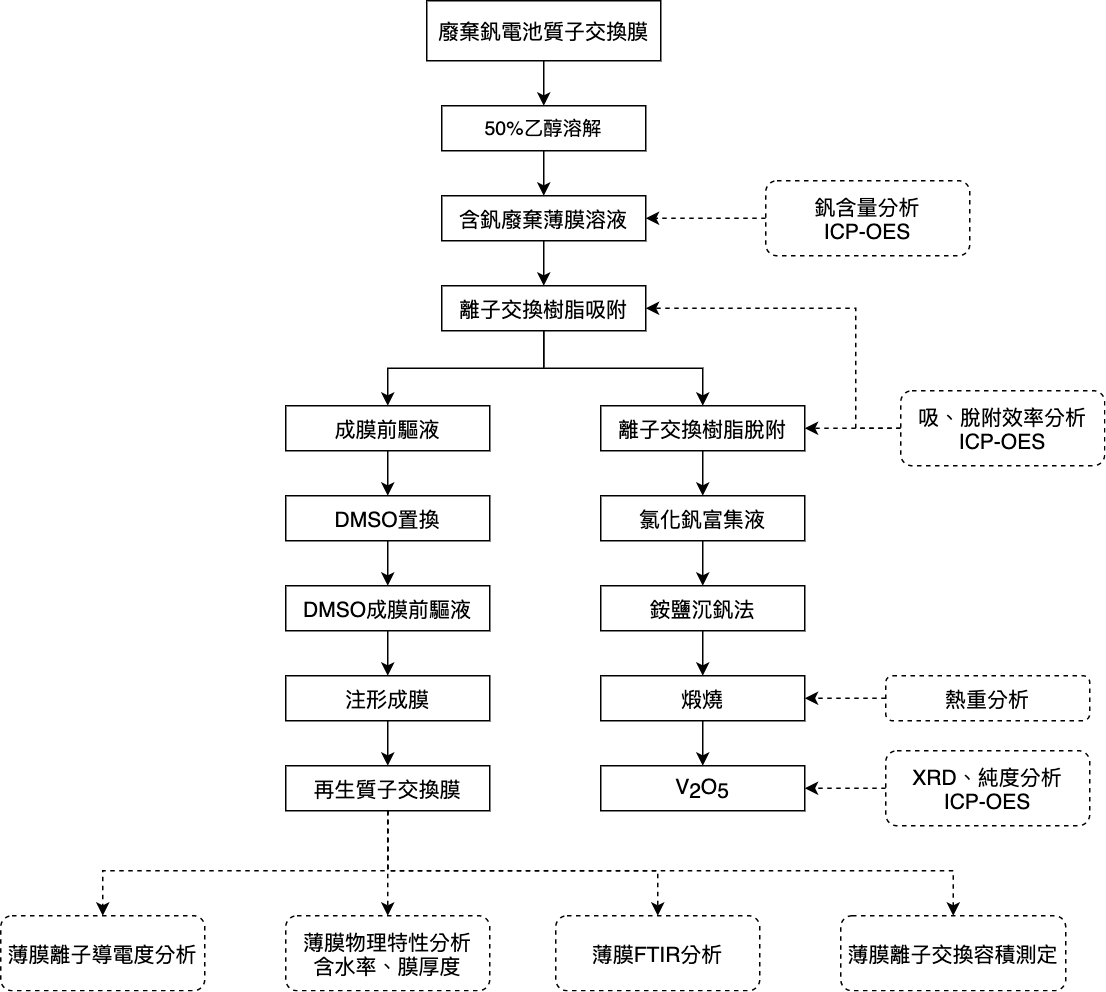
| Summary | This study aims to provide a system to recycle vanadium resourcesrecover membranes from waste proton-exchange membranes. This research is divided into two parts. To begin, ion exchange batchcolumn experiments with Dowex G26 resin were applied to adsorb vanadium in a membrane. After obtaining the vanadium-free dispersion, the recycled membrane was prepared by recasting it in the second part. |
||
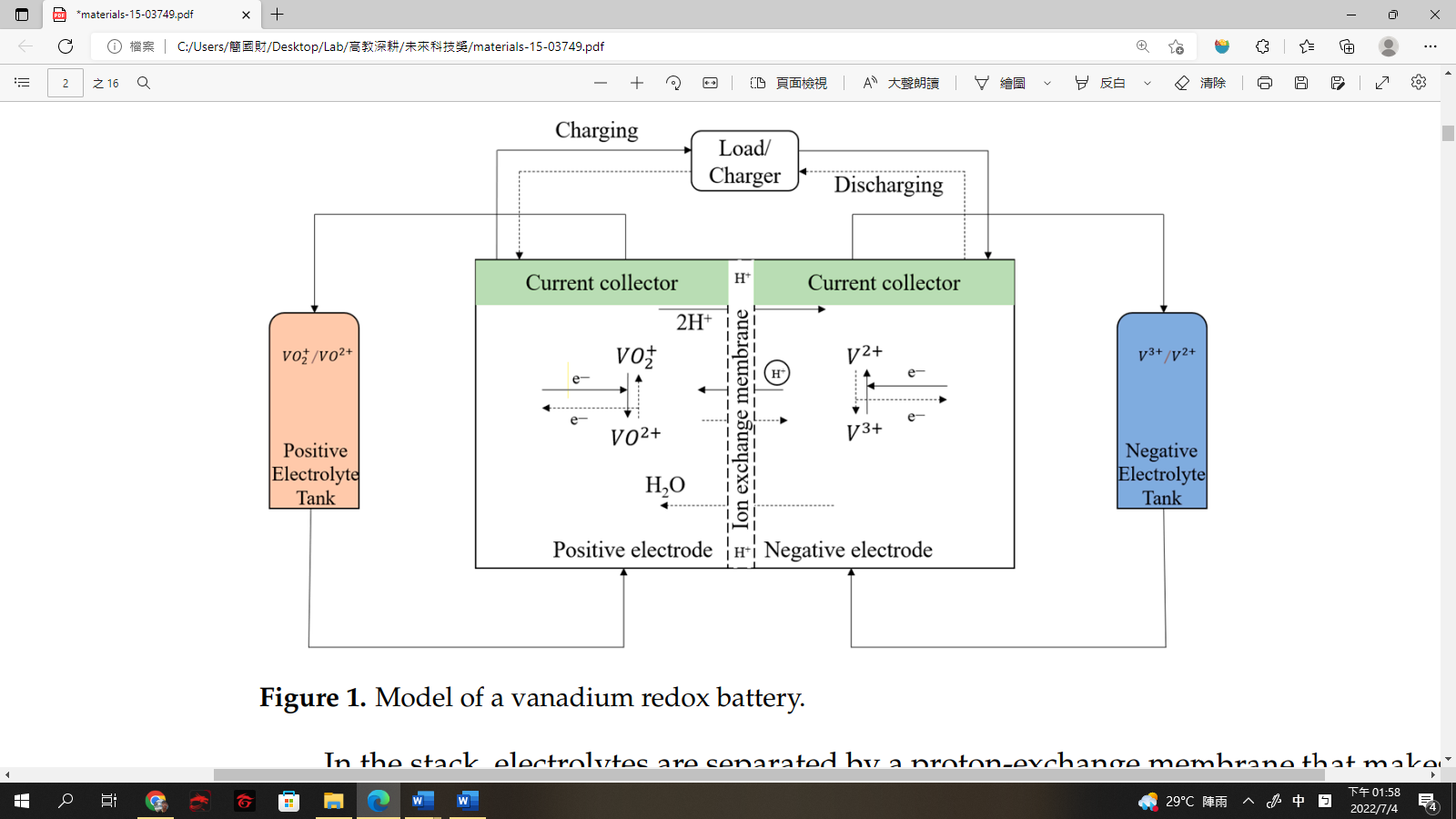
| Scientific Breakthrough | "The vanadium flow battery (VFB) has attracted considerable attention as a future energy storage system that can offer a megawatt/h storage of the electric energy from renewable energies, including solar energywind energy. The continuous decrease of conductivity will deteriorate the battery capacityfinally make the membrane dysfunctional. This scenario will cause numerous costs with replacing new membrane. Therefore, it is necessary to recover waste membranerecycle vanadium resource inside the membrane.There is no research yet reporting the method of recovering the vanadium from waste VFB membrane. This study aims to provide a sufficiencysimple method to recycle vanadium resourcerecover membrane from waste Nafion |
||
| Industrial Applicability | By using the ion-exchange method, vanadium could be removed efficiently,high purity of the vanadium product was obtained after enrichment, precipitation,calcination,this could be reused as a raw material in industry. The ion-exchange capacityion conductivity of the recycled membrane were significantly ameliorated however, there is still room for improvement to reach the same level as the commercial membrane. In summary, the vanadiummembrane were recovered simultaneously from the waste vanadium flow battery. This research has great potential toward the goal of waste reductionresource circulation. |
||
| Keyword | Fuel cell vanadium flow battery proton-exchange membrane hydrometallurgy vanadium adsorb ion exchange Dowex G26 recovery recast sustainable manufacturing | ||
- Contact
- Rita Kuo
- ritakuo@gs.ncku.edu.tw
other people also saw