| Technical Name | Next generation Saponin-based Vaccine Adjuvant | ||
|---|---|---|---|
| Project Operator | National Taiwan University School of Pharmacy | ||
| Project Host | 梁碧惠 | ||
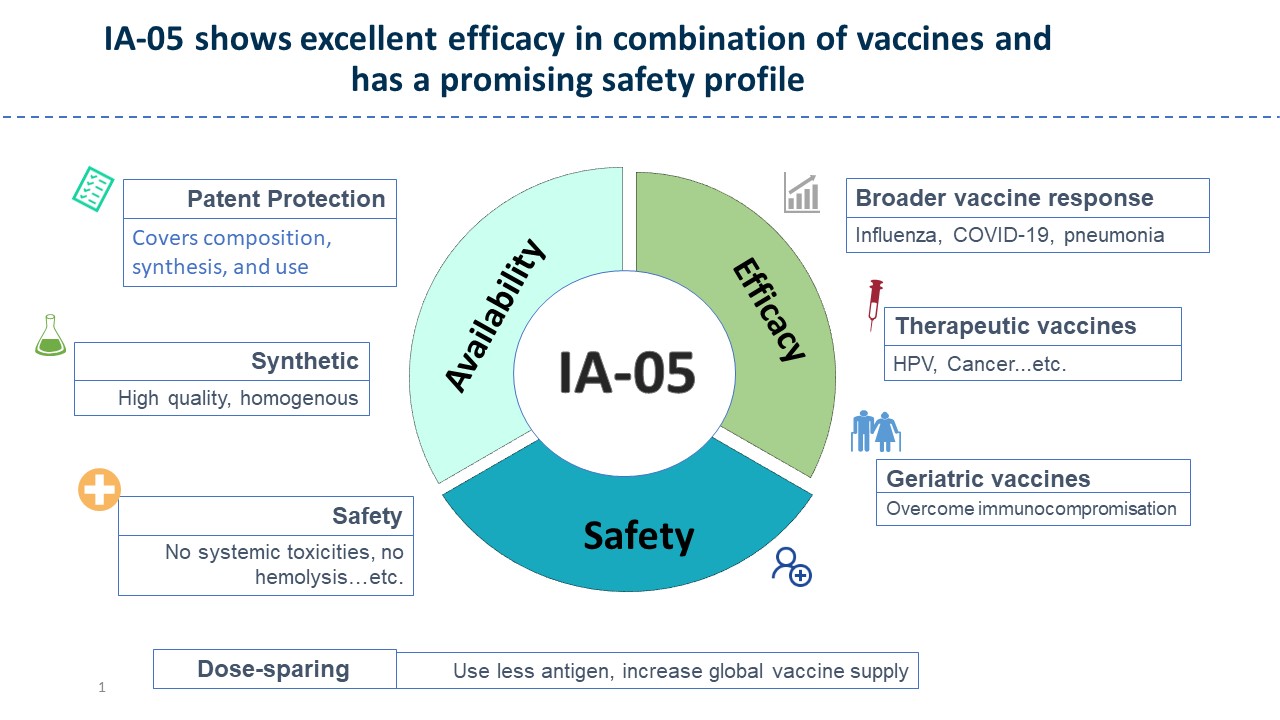
| Summary | A novel, patented, saponins-based vaccine adjuvant, IA-05, incorporated with various vaccines resulted in superior humoralcellular immunity to other adjuvants. Preliminary IA-05 safety data is compelling: in animal modelsat doses sufficient to elicit a durable immune response, IA-05 was not associated with any observable toxicity. IA-05 is a small molecule with good stability profileshigh scalability. Accordingly, IA-05 is proposed for further development in various vaccines for achieving better immune efficacyprotection. |
||
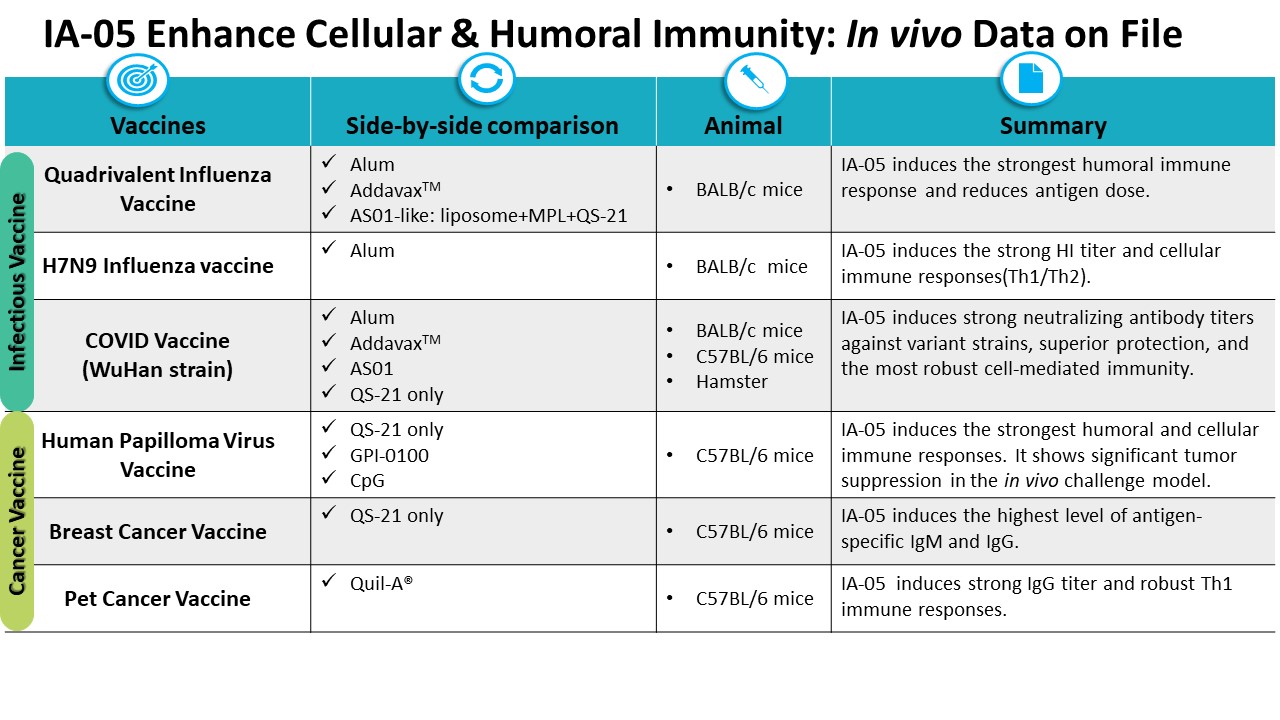
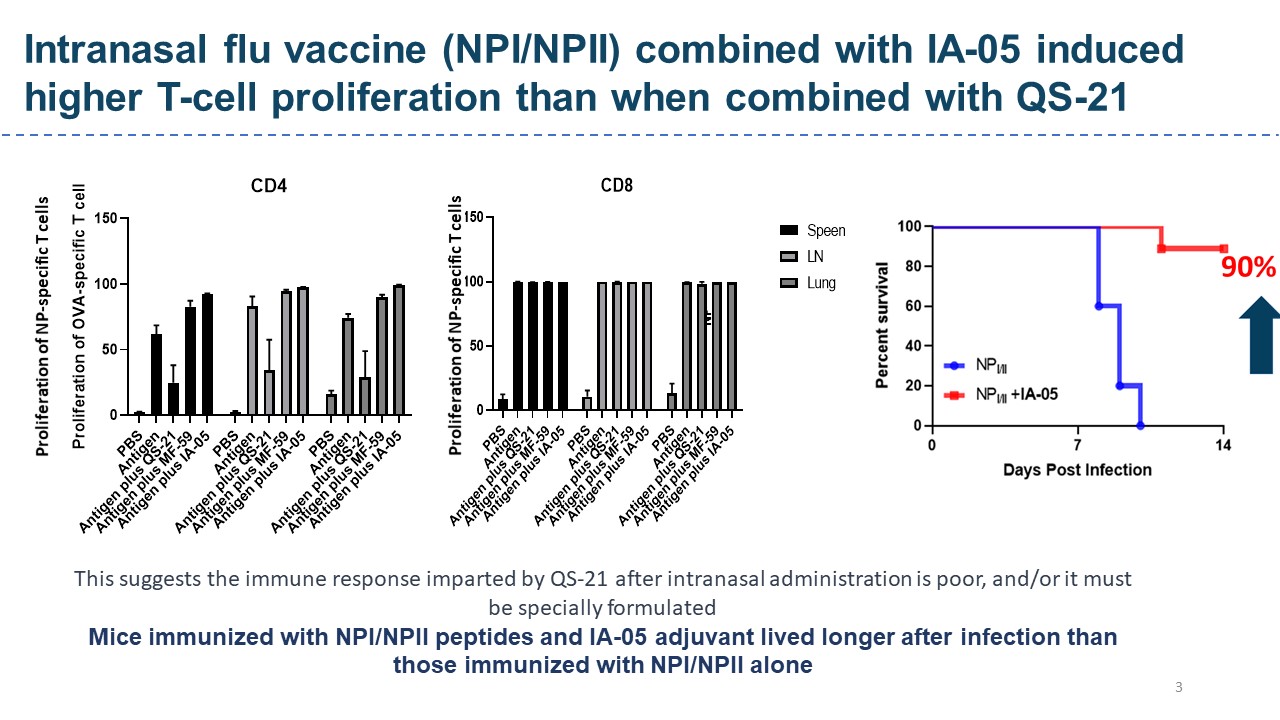
| Scientific Breakthrough | QS-21, a purified glycoside from Quillaja Saponaria, has been licensed for use as an adjuvant in herpes zostermalaria vaccines. Its widespread use is limited by its structural instabilitythe low yielding of the purification. IA-05 combined with vaccines of anti-SARS-CoV-2, anti-influenza,anti- HPV vaccines increased several-folds of humorallong-lasting cellular immunity than approved adjuvants, but no significant body weight change, nor hemolysis of IA-05 were observed. IA-05 is a rationally designed analog of QS-21 with improved stability, safety,scalability. GMP production of IA05 is completedready to conduct IND enable study. |
||
| Industrial Applicability | IA-05 is an adjuvant with a higher potential to help vaccines with better immune responseprotection. This product improves vaccine efficacyreduces the potential toxicity of the vaccine. In addition, IA-05 has high purityhigh stabilityis manufactured under GMP regulation. It is ready to enter pre-clinical IND-enabling studieswe plan to co-developout-license it to vaccine companies. If this adjuvant can successfully prove its safety in human trials, it will have the opportunity to authorize the use of several vaccines on this platformobtain continuous tech-tranfersroyalties. |
||
| Keyword | vaccine adjuvant immune stimulating COVID vaccine QS-21 皂苷 anti-cancer vaccines vaccine protection | ||
- Contact
- Pi-Hui Liang
- phliang@ntu.edu.tw
other people also saw