
prev
next
| Technical Name | 子宮內膜癌安蓓甲基化基因檢測 | ||
|---|---|---|---|
| Project Operator | Taipei Medical University | ||
| Project Host | 賴鴻政 | ||
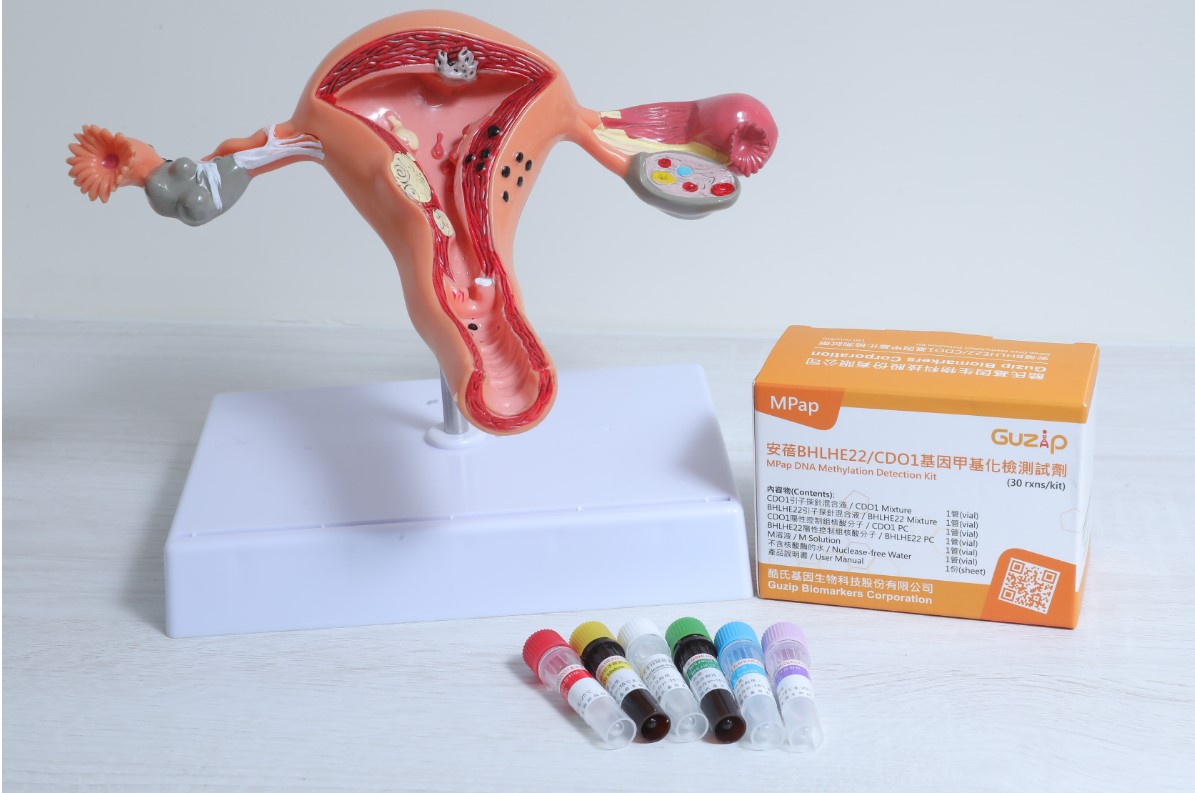
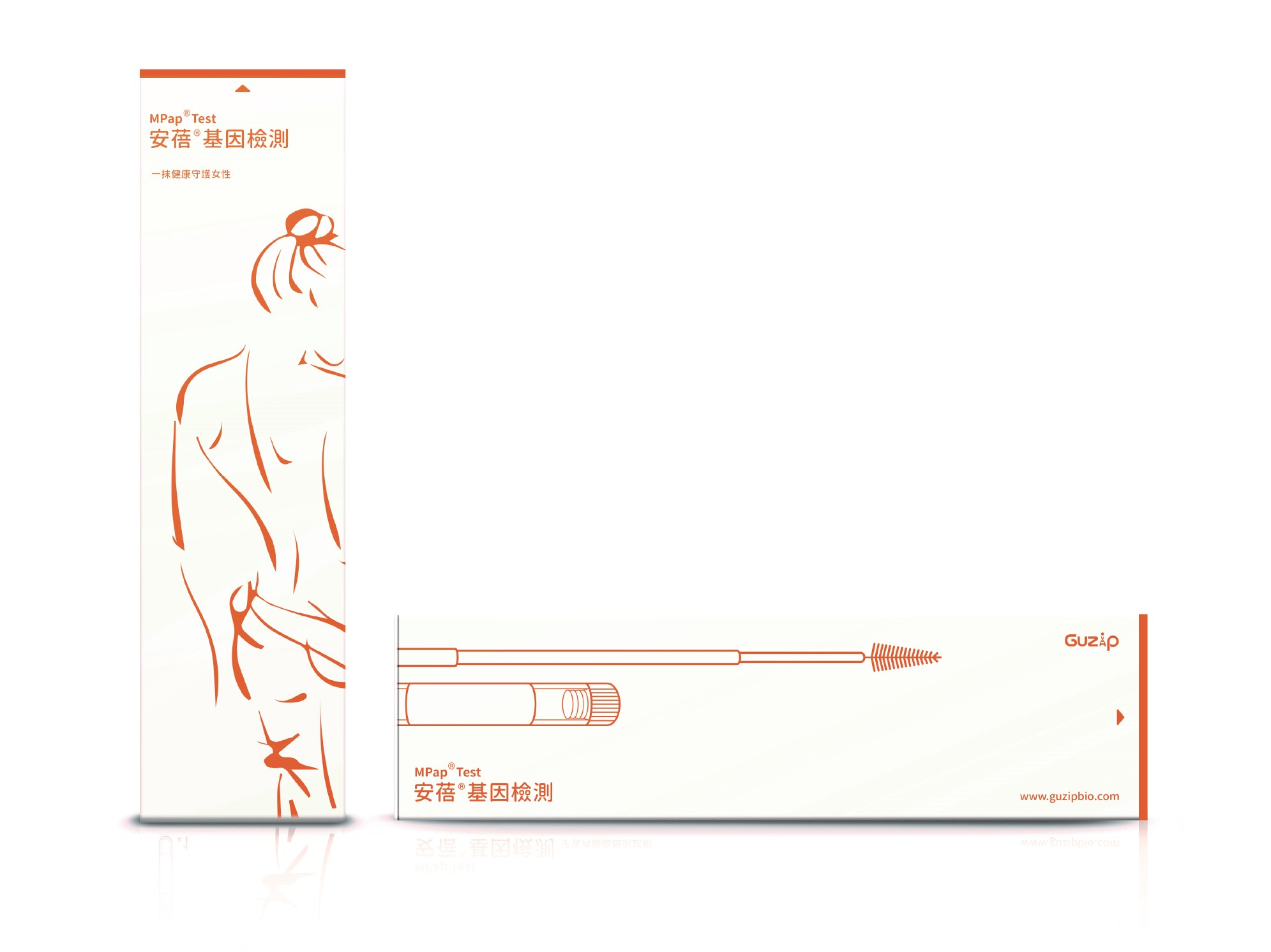
| Summary | The MPap DNA Methylation Test is an in vitro diagnostic reagent for endometrial cancer detection. For women atover 40 years of age with abnormal uterine bleeding, the methylation status of BHLHE22CDO1 genes from conventional Pap smear, can be used as an auxiliary diagnosis of endometrial cancer. The test result provides an important triage for further invasive endometrial biopsy, which will substantially reduce the need of invasive proceduresincrease the feasibility of endometrial cancer screening in high risk populations. This is a ground breaking test. |
||
| Technical Film | |||
| Scientific Breakthrough | There is no molecular testing for endometrial cancer detection so far. The MPap DNA Test, based on DNA methylation from Pap smear, is the first in class product. From a clinical trial of 5 medical centers in Taiwan in 2018-2020, MPap, with sensitivity, specificity, PPVNPV of 92.5, 73.8, 40.2,98.1, respectively, all outperformed transvaginal ultrasound. This is the first multicenter study to validate the performance of methylation biomarker-based testing for EC detectionperform a head-to-head comparison of such testing with transvaginal ultrasound. |
||
| Industrial Applicability | The MPap Test broadens the scope of conventional Pap smearis the first-in-market DNA methylation biomarker of endometrial cancer for women with abnormal uterine bleeding. MPap provides physician a reliable triage reference for further invasive proceduresnot with high sensitivityspecificity, which will substantially reduce the riskcost of women’s health care. The easecomfort of diagnosing process will also improve the feasibility of endometrial cancer detection. |
||
| Matching Needs | 天使投資人、策略合作夥伴 |
||
| Keyword | DNA methylation endometrial cancer in vitro diagnostics Pap smear abnormal uterine bleeding MPap | ||
- gyntsgh2@gmail.com
other people also saw
prev
next