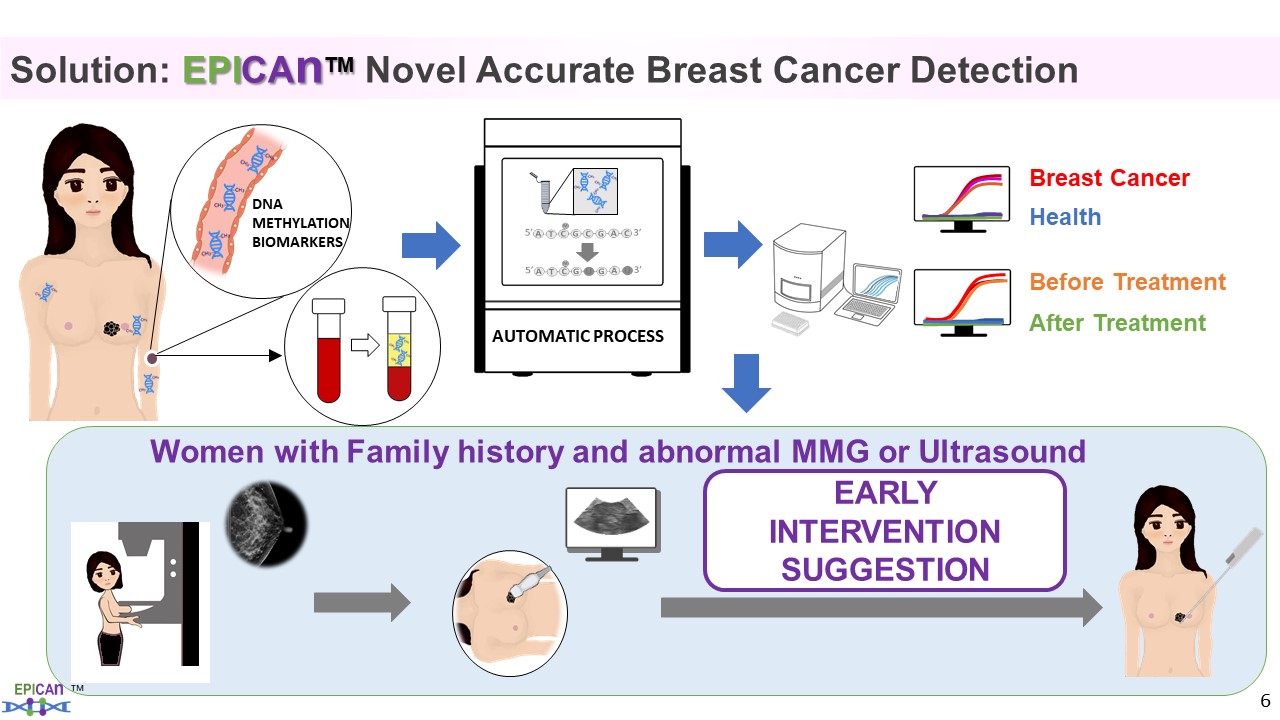
:::
- Home
- /
- Year
- /
- 2021
- /
- Interdisciplinary Area
- /
- 定量新冠病毒中和抗體創新技術
| Technical Name | 定量新冠病毒中和抗體創新技術 | ||
|---|---|---|---|
| Project Operator | Chang Gung University | ||
| Project Host | 施信如 | ||
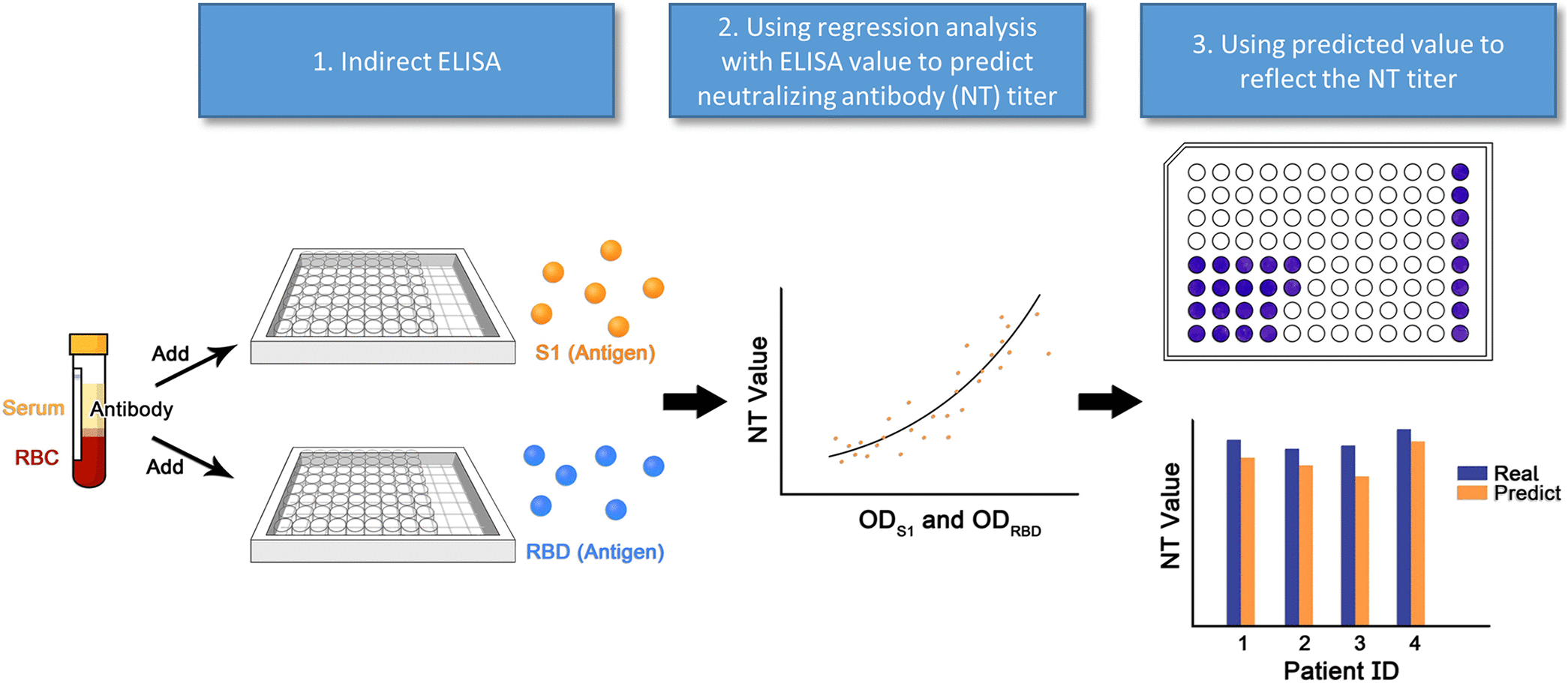
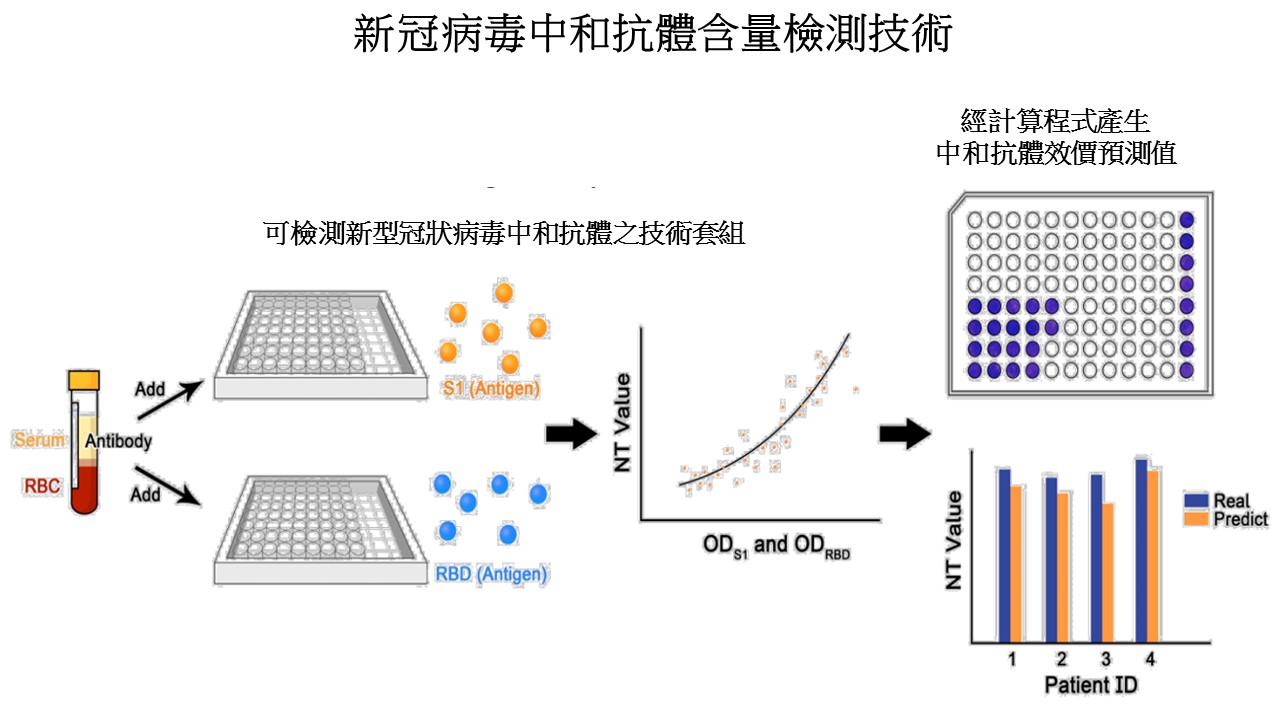
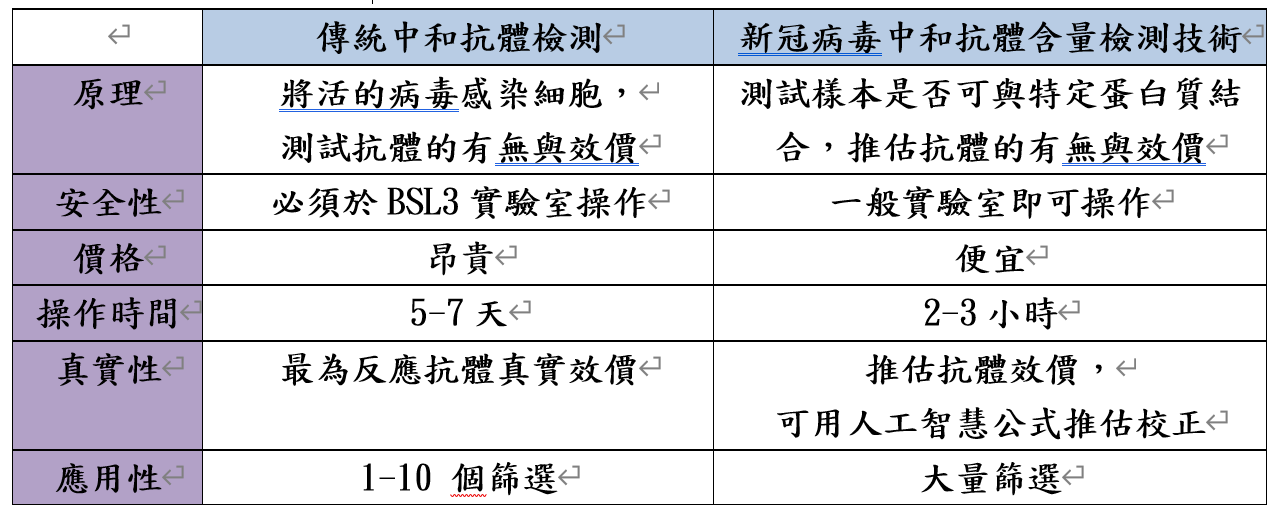
| Summary | A virus NT was performed in a biosafety level 3 laboratory to determine the titers. A SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) was designed to detect neutralizing antibodies in the serum, based on the binding affinity of SARS-CoV-2 viral protein 1viral protein 2 to antibodies. ELISA results viral protein 12, generated comparable neutralizing antibody titers. The results analyzed by spline regressiontwo-variable generalized additive model precisely reflected the real NT value. Our method can serve as a surrogate to quantify neutralizing antibody titer. |
||
| Scientific Breakthrough | This technology serves as a surrogate to quantifying neutralizing antibody titer which should be performed at P3 laboratory for several days. The new method can be performed at a general laboratorycan obtain the results within 2-3 hours, which has great potential application for clinical use to assess vaccine efficacy. |
||
| Industrial Applicability | The technology can measure the amount of neutralizing antibody which correlates to protection efficacy of an individual whether he/she would be infected again by COVID-19. This diagnostic kit will help vaccine efficacy validationhas a great potential to provide laboratory data for so-called “immunity passport”. |
||
| Matching Needs | 天使投資人、策略合作夥伴 |
||
| Keyword | SARS-CoV-2 Neutralization Antibody ELISA | ||
- mark1005@gmail.com