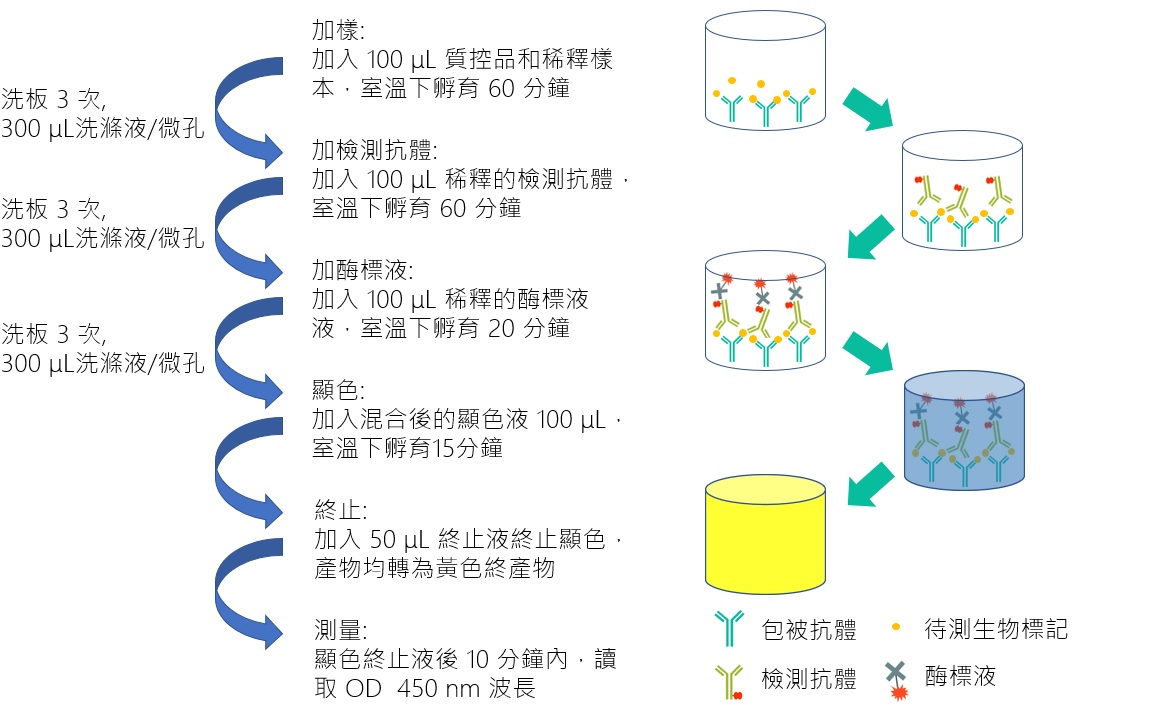
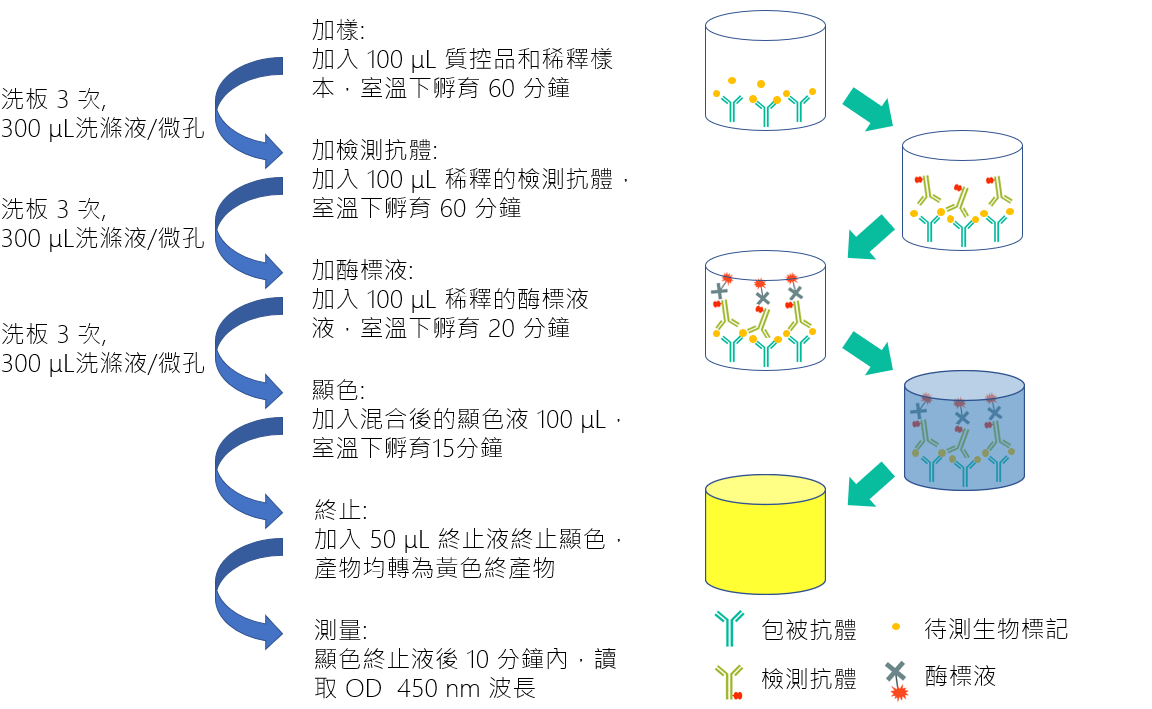
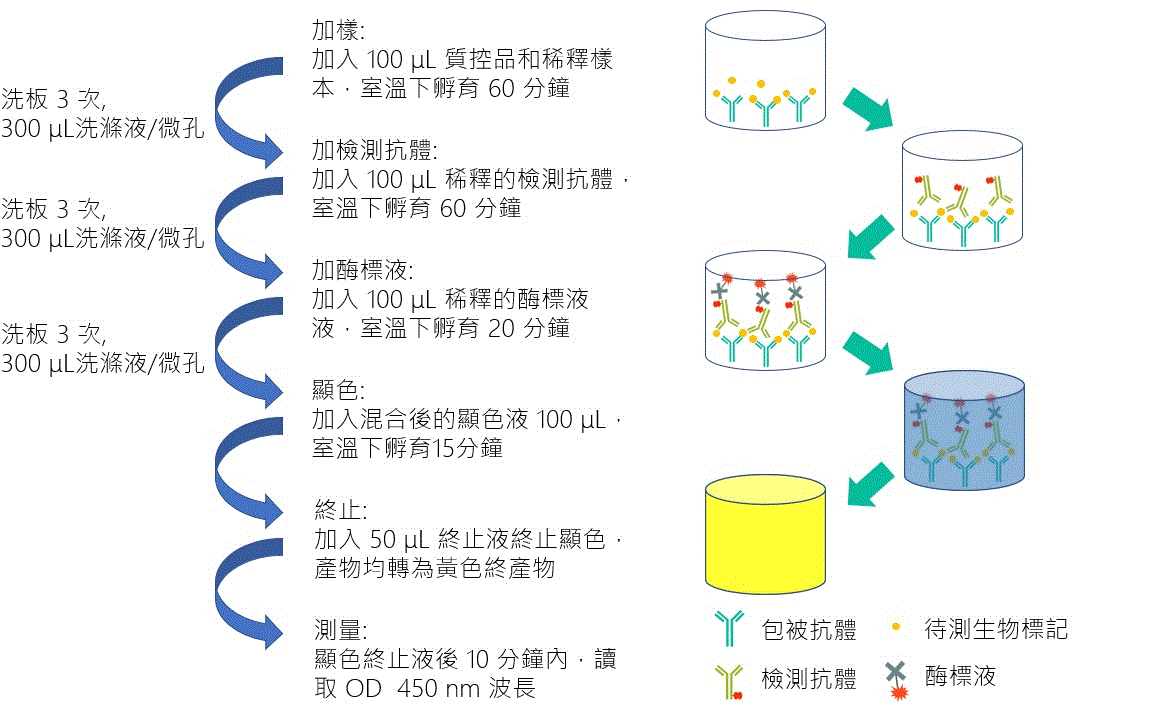
| Technical Name | ELISA Based Kawasaki Disease Biomarker In Vitro Diagnostic Devices | ||
|---|---|---|---|
| Project Operator | Academia Sinica | ||
| Project Host | 鄔哲源 | ||
| Summary | Kawasaki disease (KD), a multisystem inflammatory condition observed in younger children, can cause acute vasculitis, most notably affecting the coronary arteries. Without treatment, approximately 20-25% of children with KD develop coronary artery abnormalities (CAAs). Intravenous immunoglobulin (IVIG) treatment can reduce the incidence of CAAs to approximately 5%, but early detection is necessary. KD diagnosis is difficult, especially at the early stage. Currently, KD diagnosis is based on clinical symptoms. However, overlapping clinical features and laboratory parameters between KD and other conditions make definitive diagnosis difficult, and no specific laboratory tests are available. Therefore, identification of specific biomarkers to facilitate KD diagnosis by laboratory analysis would be valuable for preventing serious KD sequelae, especially CAAs. This kit is used for detecting protein levels of IP-10 in blood, which is a novel common biosignature of acute KD. |
||
| Scientific Breakthrough | Early diagnosis and treatment is crucial if the damage is to be limited. Our previous study has identified chemokine CXCL10/IP-10 as a protein biomarker for clinical diagnosis. Here, we develop an enzyme-linked immunosorbent assay (ELISA)-based clinical diagnostic kit for the detection of chemokine CXCL10/IP-10. This diagnostic kit is expected to ease and expedite KD's diagnosis and helps to reduce the incidence of CAAs in KD populations. The study will present an insight into novel diagnostic strategies promoting early and definitive detection of KD. |
||
| Industrial Applicability | Here we developed a new kit for early diagnosis of Kawasaki disease in clinical practice by detecting the levels of IP-10. When IP-10 exceeds the cut-off value, the diagnosis of Kawasaki disease is determined. It can significantly reduce the situation of inferior polarity leading to delayed treatment. The detection range of biomarker is 62.5-4000 pg/mL, the quantification concentration can be up to 100 pg/mL with highly specific antibody, and the intra-batch precision %CV is less than 10%. The operation process is simple and fast. It only takes 4 hours to complete the test without waiting until the next day. For the treatment of Kawasaki children with time-sensitive, it can be judged early whether they need special drug treatment, and it has high clinical value in early diagnosis. |
||
| Keyword | enzyme-linked immunosorbent assay (ELISA) Interferon (IFN)-γ inducible protein-10 Myeloid progenitor inhibitory factor 1 In vitro diagnostic devices Kawasaki disease blood biosignature coronary artery aneurysm Intravenous Immunoglobulin incomplete Kawasaki disease acquired heart disease | ||
- htliao@ibms.sinica.edu.tw
other people also saw