| Technical Name |
Low-dose nanoscale biomimetic cell structure – Next-generation platform technology for advanced precision immunotherapy |
| Project Operator |
China Medical University Hospital |
| Project Host |
陳三元 |
| Summary |
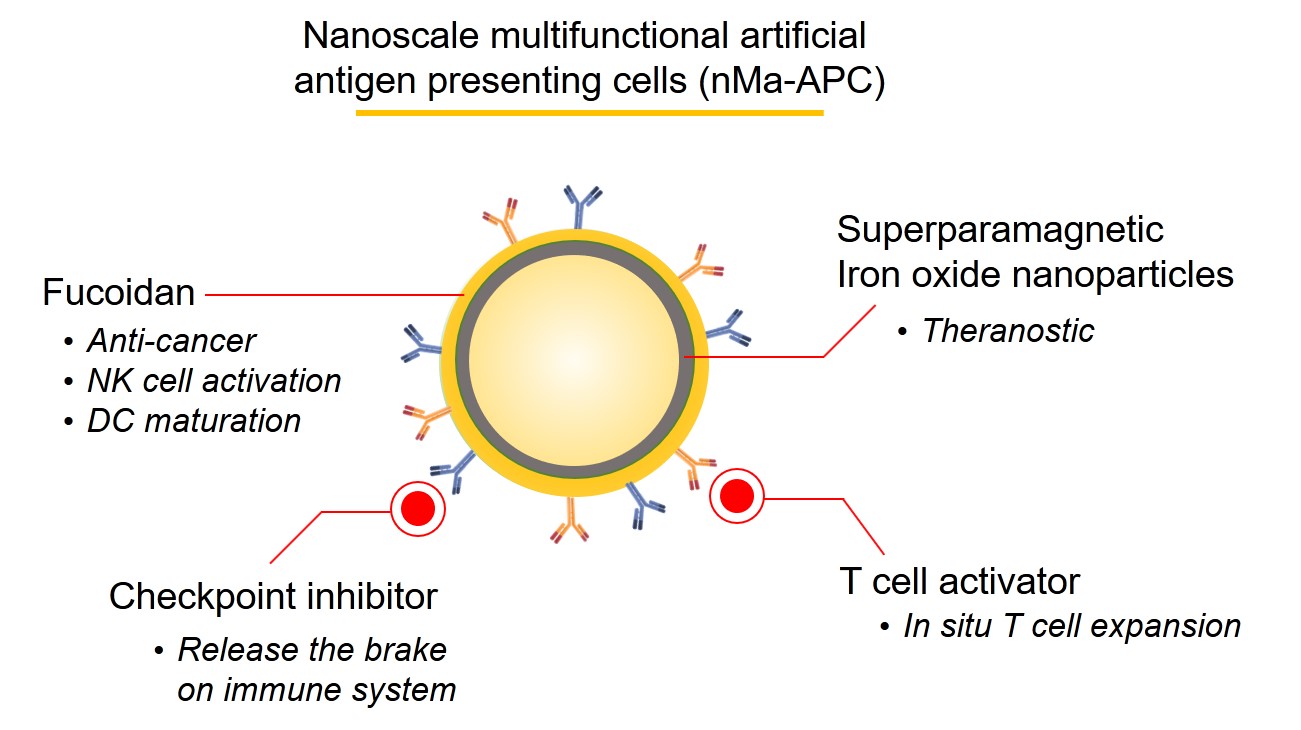
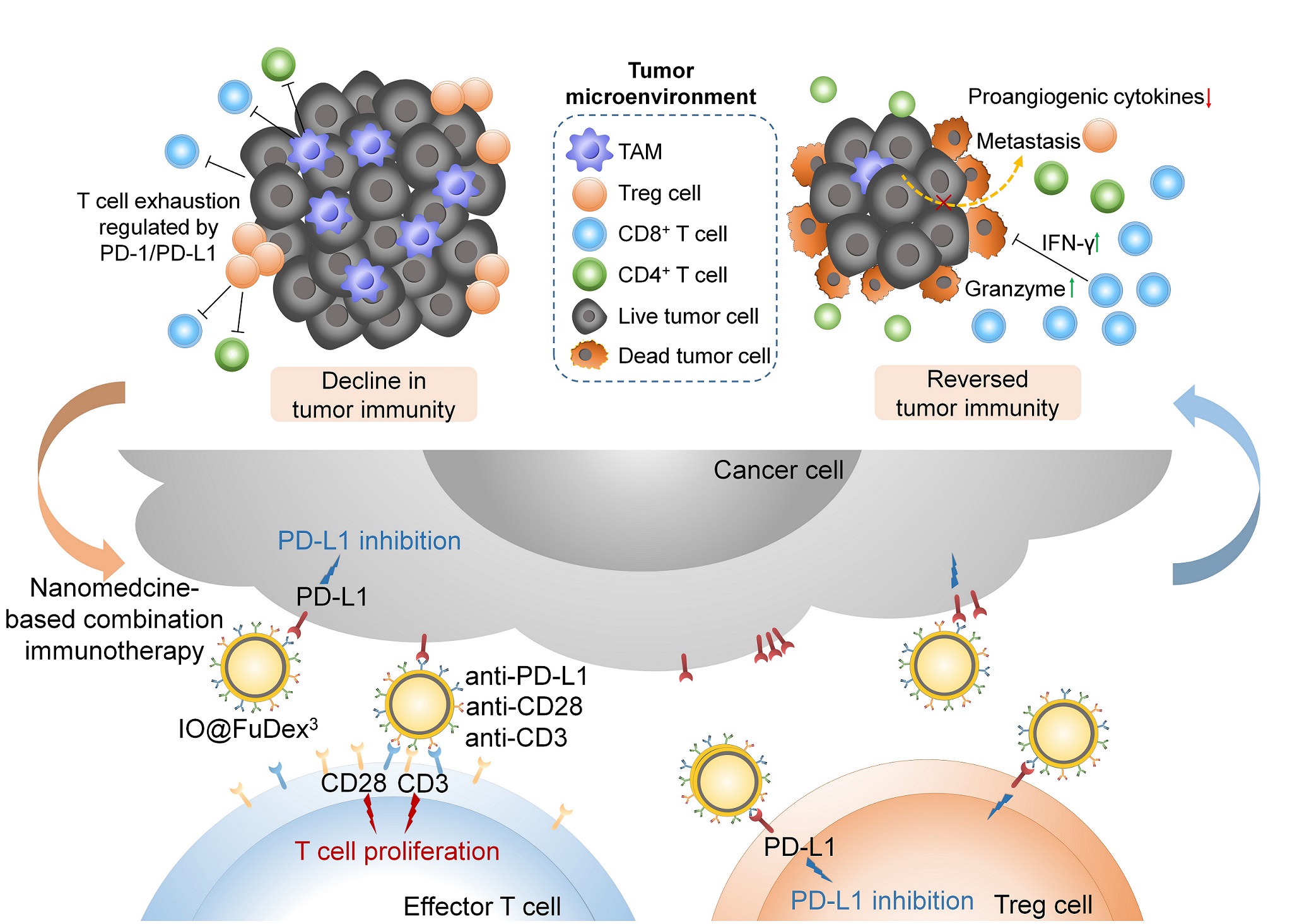
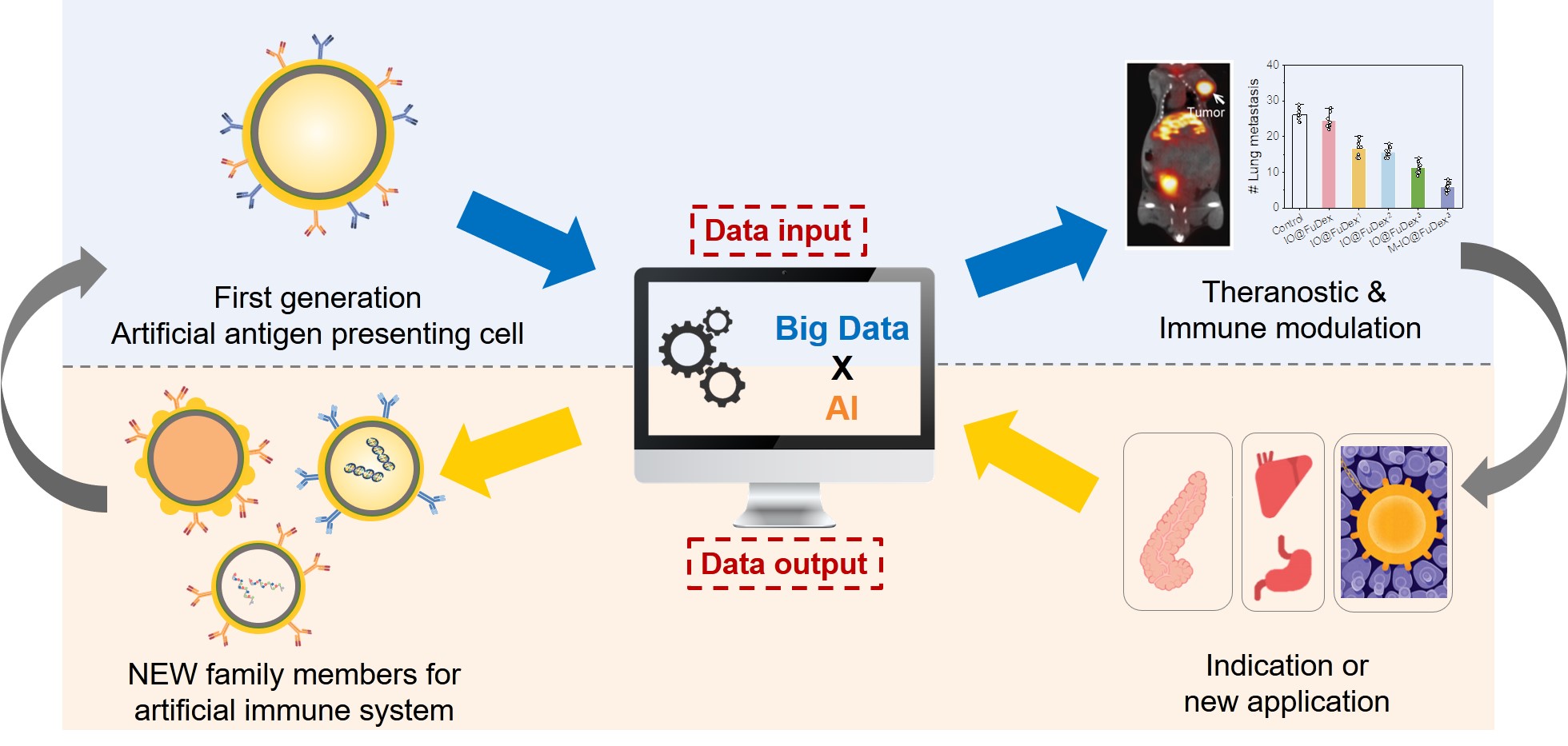
We developed a biomimetic triple-antibody-immobilized magnetic fucoidan nanomedicine as a multifunctional artificial antigen presenting cell, which possessed the ability to not only inhibit immune checkpoint but activate tumor infiltrated T cells. of Bridging sites with tunable density on the nanoplatform was designed, allowing the antibodies to be well-distributed on the surface for mimicking immune cells. In contrast to the
complex cell expansion process using microbeads in adaptive cell
therapy, the nanoplatform can be i.v. administrated to cut the course of
therapy from several weeks to days. With the development of the
platform technology, an artificial immune system family can be built to
pave the way for personalized immunotherapy. |
| Scientific Breakthrough |
We developed the nanoscale multifunctional artificial antigen presenting cells (nMa-APC) for renovating immunotherapy. nMa-APC with the presence of well-distributed antibodies on the surface achieve in-situ T cell expansionimmune brake release simultaneously. Compared with natural APCs, nMa-APC represents a cutting-edge strategy to activate multiple anti-tumor immune pathways . Now, nMa-APC targeting different mechanisms of action shows superior therapeutic efficacy compared with checkpoint immunotherapy. Additionally, nMa-APC possesses huge potential to take over the market of adaptive immunotherapy as the course of treatment can be cut from several weeks to days. Based on the platform technology, we expect to establish an artificial immune system for personalized therapy. |
| Industrial Applicability |
The disclosed nanoscale multifunctional artificial antigen presenting cells (nMa-APC) that simultaneously targets various mechanism of actions can boost the anticancer immune responses. Compared with checkpoint immunotherapy, nMa-APC offers not only brake release of immune system but T cell expansion to augment therapeutic efficacy. Moreover, the substantially reduced course of treatment from several weeks to days indicates the superiority of nMa-APC to adaptive cell therapy. As the revenues of OPDIVO® listed 7,200 million at 2019, nMa-APC is expected to penetrate the market with respectable potential. Additionally, the platform technology allows the efficient establishment of pipelines to achieve artificial immune system, making personalized immunotherapy in the foreseeable future |
| Keyword |
Immunotherapy Checkpoint inhibitor Adaptive cell therapy Artificial antigen presenting cell Fucoidan Personalized therapy Nanotechnology Bionic Magnetic targeting Theranostic |